Unit 1 Moles 7 1 The Mole Collections






- Slides: 16

Unit 1 Moles 7. 1 The Mole Collections of items include dozen, gross, and mole. 1 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Collection Terms A collection term states a specific number of items. • 1 dozen donuts = 12 donuts • 1 ream of paper = 500 sheets • 1 case = 24 cans Collections of items include dozen, gross, and mole. 2 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

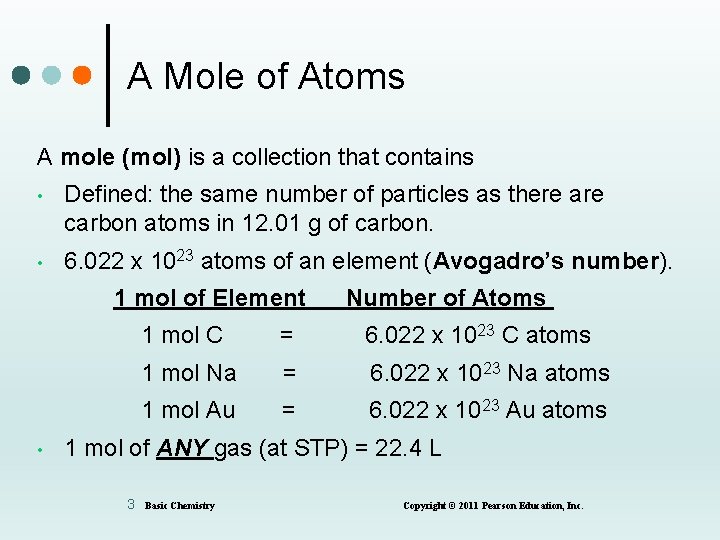
A Mole of Atoms A mole (mol) is a collection that contains • Defined: the same number of particles as there are carbon atoms in 12. 01 g of carbon. • 6. 022 x 1023 atoms of an element (Avogadro’s number). 1 mol of Element • Number of Atoms 1 mol C = 6. 022 x 1023 C atoms 1 mol Na = 6. 022 x 1023 Na atoms 1 mol Au = 6. 022 x 1023 Au atoms 1 mol of ANY gas (at STP) = 22. 4 L 3 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

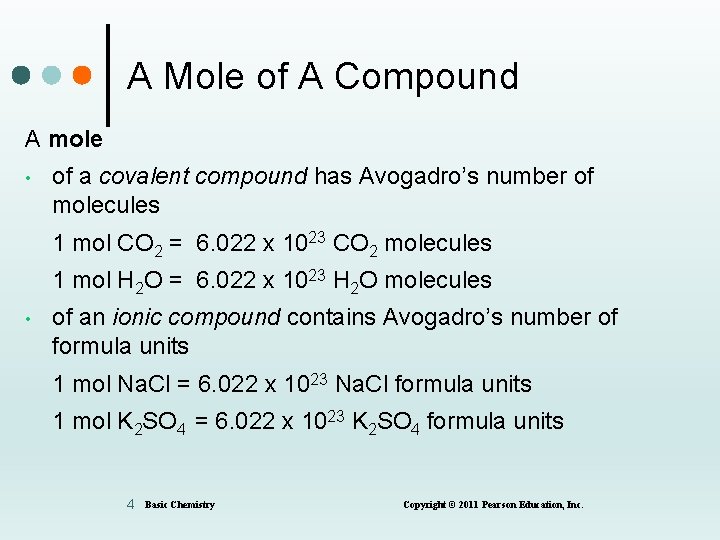
A Mole of A Compound A mole • of a covalent compound has Avogadro’s number of molecules 1 mol CO 2 = 6. 022 x 1023 CO 2 molecules 1 mol H 2 O = 6. 022 x 1023 H 2 O molecules • of an ionic compound contains Avogadro’s number of formula units 1 mol Na. Cl = 6. 022 x 1023 Na. Cl formula units 1 mol K 2 SO 4 = 6. 022 x 1023 K 2 SO 4 formula units 4 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

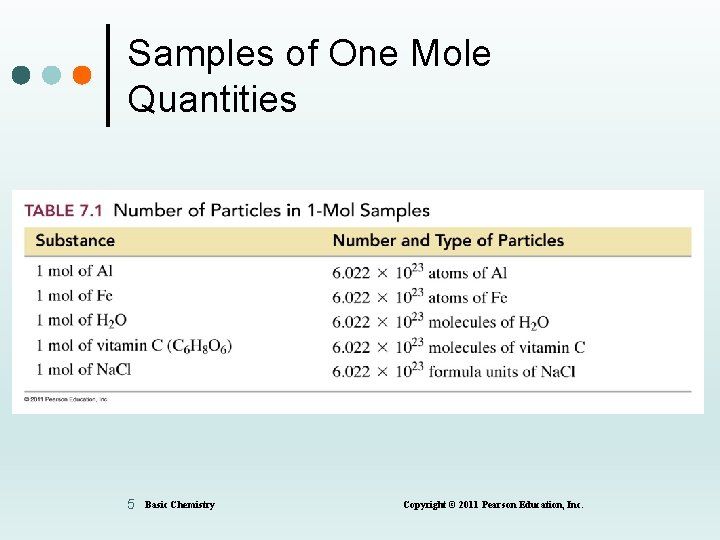
Samples of One Mole Quantities 5 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Avogadro’s Number Avogadro’s number (6. 022 x 1023) can be written as an equality and two conversion factors. Equality: 1 mol = 6. 022 x 1023 particles Conversion Factors: (dimensional analysis) 6. 022 x 1023 particles and 1 mol 6. 022 x 1023 particles 6 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Using Avogadro’s Number Avogadro’s number converts moles of a substance to the number of particles. How many Cu atoms are in 0. 50 mol of Cu? 0. 50 mol Cu x 6. 022 x 1023 Cu atoms 1 mol Cu (measured) (definition) = 3. 0 x 1023 Cu atoms 7 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

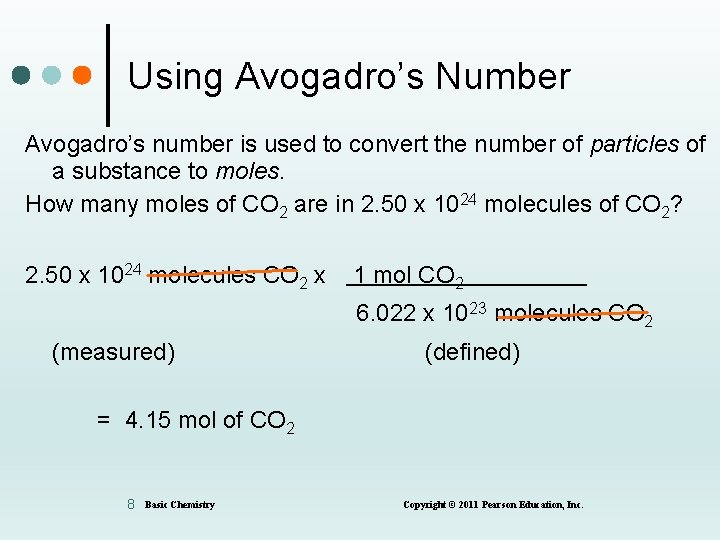
Using Avogadro’s Number Avogadro’s number is used to convert the number of particles of a substance to moles. How many moles of CO 2 are in 2. 50 x 1024 molecules of CO 2? 2. 50 x 1024 molecules CO 2 x 1 mol CO 2 6. 022 x 10 23 molecules CO 2 (measured) (defined) = 4. 15 mol of CO 2 8 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

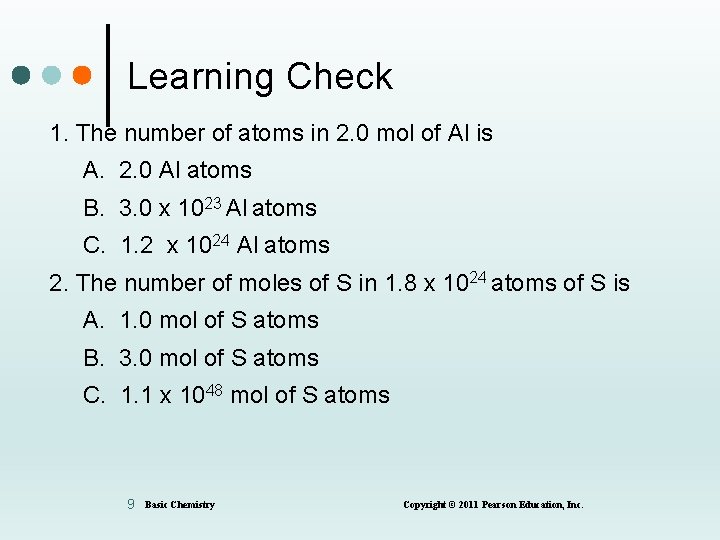
Learning Check 1. The number of atoms in 2. 0 mol of Al is A. 2. 0 Al atoms B. 3. 0 x 1023 Al atoms C. 1. 2 x 1024 Al atoms 2. The number of moles of S in 1. 8 x 1024 atoms of S is A. 1. 0 mol of S atoms B. 3. 0 mol of S atoms C. 1. 1 x 1048 mol of S atoms 9 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

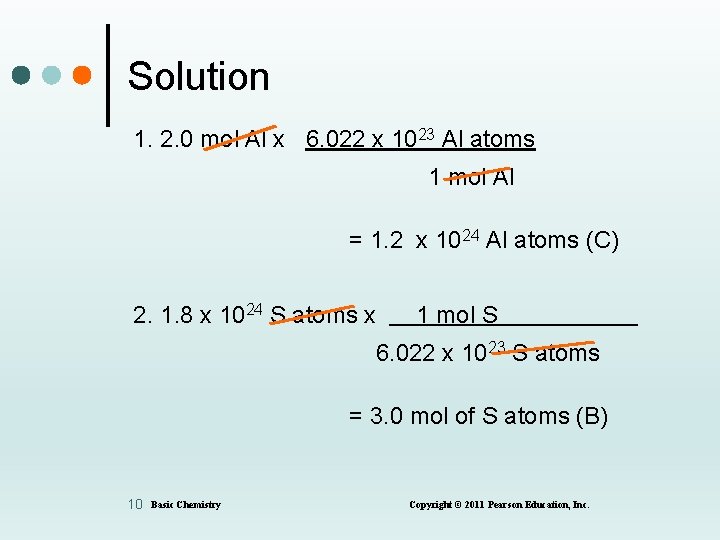
Solution 1. 2. 0 mol Al x 6. 022 x 1023 Al atoms 1 mol Al = 1. 2 x 1024 Al atoms (C) 2. 1. 8 x 1024 S atoms x 1 mol S 6. 022 x 1023 S atoms = 3. 0 mol of S atoms (B) 10 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Subscripts and Moles The subscripts in a formula tells us: • the relationship of atoms in the formula • the moles of each element in 1 mol of compound Glucose C 6 H 12 O 6 1 molecule: 6 atoms of C 1 mol: 6 mol of C 12 atoms of H 12 mol of H 6 atoms of O 6 mol of O If we had 3 bicycles, how many wheels would we have? 11 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

Subscripts State Atoms and Moles 12 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

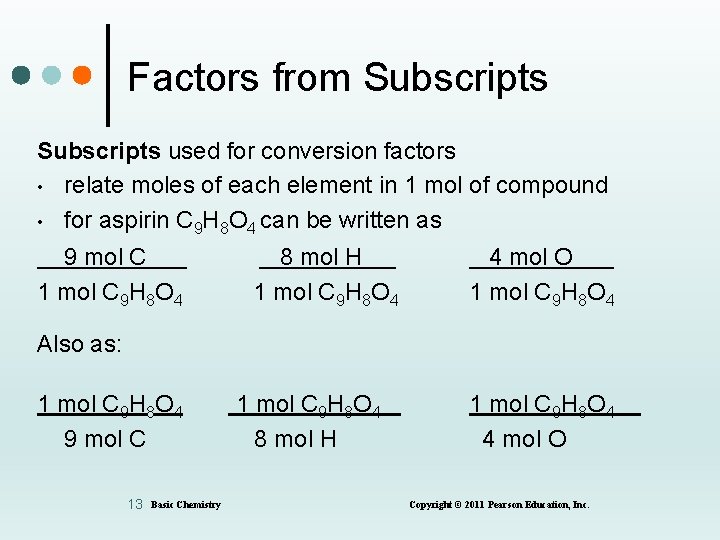
Factors from Subscripts used for conversion factors • relate moles of each element in 1 mol of compound • for aspirin C 9 H 8 O 4 can be written as 9 mol C 1 mol C 9 H 8 O 4 8 mol H 1 mol C 9 H 8 O 4 4 mol O 1 mol C 9 H 8 O 4 Also as: 1 mol C 9 H 8 O 4 9 mol C 13 Basic Chemistry 1 mol C 9 H 8 O 4 8 mol H 1 mol C 9 H 8 O 4 4 mol O Copyright © 2011 Pearson Education, Inc.

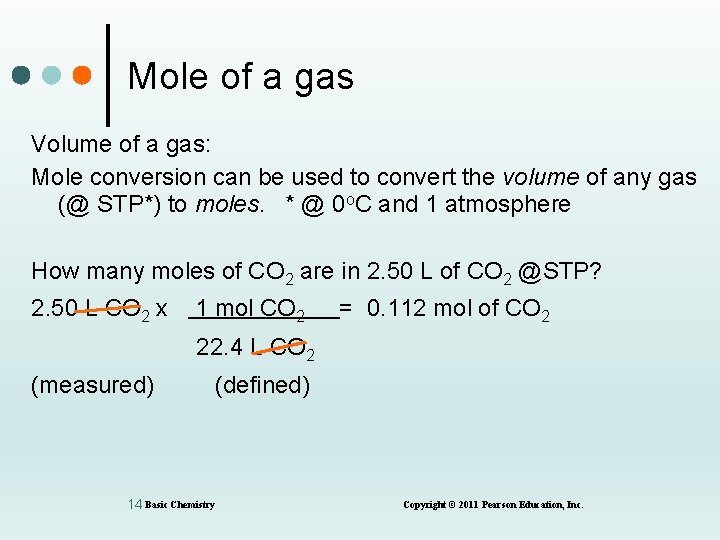
Mole of a gas Volume of a gas: Mole conversion can be used to convert the volume of any gas (@ STP*) to moles. * @ 0 o. C and 1 atmosphere How many moles of CO 2 are in 2. 50 L of CO 2 @STP? 2. 50 L CO 2 x 1 mol CO 2 = 0. 112 mol of CO 2 22. 4 L CO 2 (measured) (defined) 14 Basic Chemistry Copyright © 2011 Pearson Education, Inc.

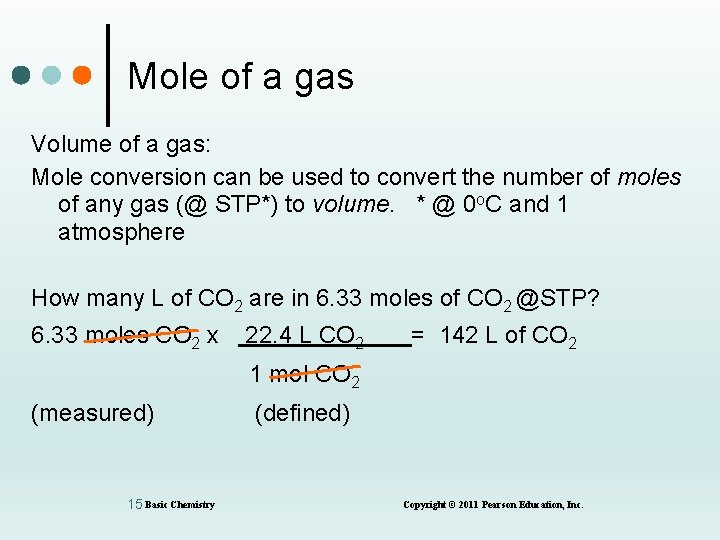
Mole of a gas Volume of a gas: Mole conversion can be used to convert the number of moles of any gas (@ STP*) to volume. * @ 0 o. C and 1 atmosphere How many L of CO 2 are in 6. 33 moles of CO 2 @STP? 6. 33 moles CO 2 x 22. 4 L CO 2 = 142 L of CO 2 1 mol CO 2 (measured) 15 Basic Chemistry (defined) Copyright © 2011 Pearson Education, Inc.

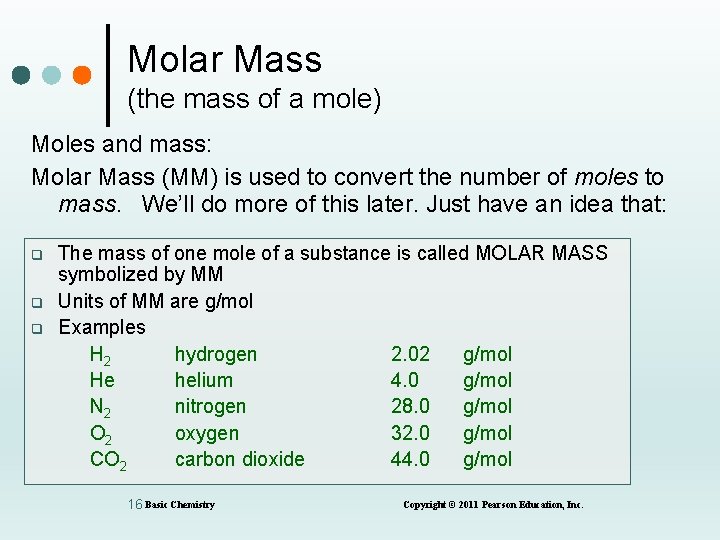
Molar Mass (the mass of a mole) Moles and mass: Molar Mass (MM) is used to convert the number of moles to mass. We’ll do more of this later. Just have an idea that: q q q The mass of one mole of a substance is called MOLAR MASS symbolized by MM Units of MM are g/mol Examples H 2 hydrogen 2. 02 g/mol He helium 4. 0 g/mol N 2 nitrogen 28. 0 g/mol O 2 oxygen 32. 0 g/mol CO 2 carbon dioxide 44. 0 g/mol 16 Basic Chemistry Copyright © 2011 Pearson Education, Inc.