UNIT 1 Measurements and Graphing Safety During this


















- Slides: 29

UNIT #1: Measurements and Graphing

Safety During this year we will be conducting lab experiments. It is important to be safe while you are completing these labs. No running Place belongings out of the way Wear close-toed shoes Wear safety goggles if necessary Be aware of your surroundings Keep the volume down

Observations Interaction of one or more of the 5 senses with the environment. What are the five senses? 1 2 3 4 5 Make an observation about Garfield

Instruments What are used to enhance observations. are some instruments you can think of that could help in making observations?

Observation vs Inference An observation is something that can be concluded in the PRESENT An inference is an interpretation of one or more observations Inferences are hypotheses about the PAST or FUTURE Make an inference about Garfield.

Classification Organizing our observations in a meaningful way Everybody Up!

Measurement A direct comparison with a KNOWN STANDARD When we measure we must use units (No Naked Numbers) Science uses the Metric System Time: Length: Mass:

The Metric System Why would we use the metric system in science? Kilo, Hecto, Deka, Unit, Deci, Centi

The Metric Ladder



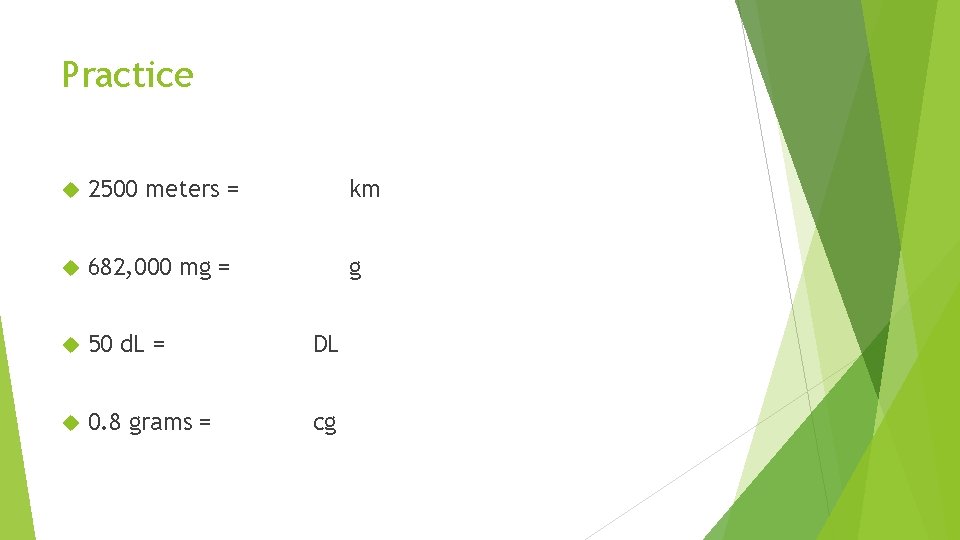
Practice 2500 meters = km 682, 000 mg = g 50 d. L = DL 0. 8 grams = cg

Scientific Notation Sometimes scientist’s measure things and get numbers that are too big or too small to write out. We use mathematical shorthand to express these numbers. Numbers like 1 Googol. (1 with 100 zeros)

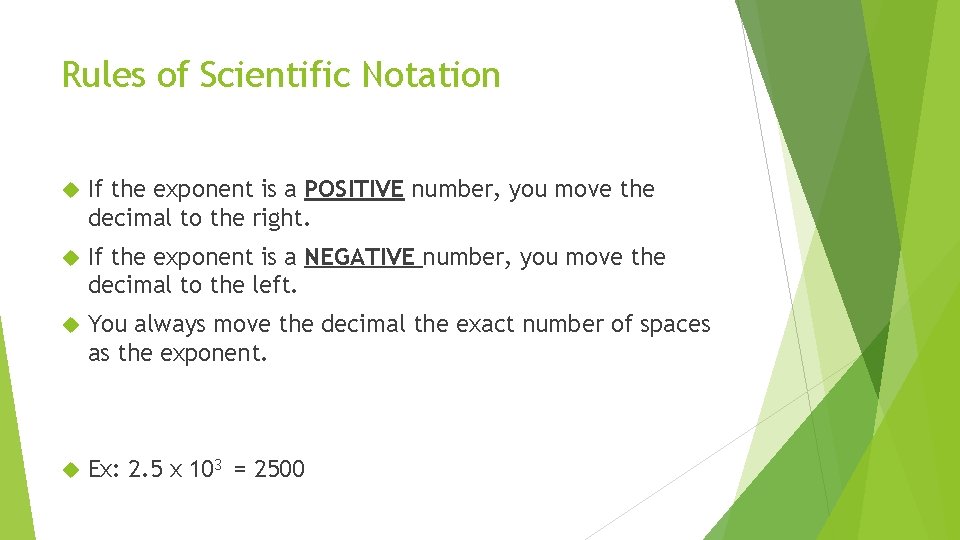
Rules of Scientific Notation If the exponent is a POSITIVE number, you move the decimal to the right. If the exponent is a NEGATIVE number, you move the decimal to the left. You always move the decimal the exact number of spaces as the exponent. Ex: 2. 5 x 103 = 2500

Expansion Practice 1. 15 x 106 9. 99 x 104 5. 6464 x 10 -2 2. 3456 x 102

Rules of Scientific Notation When contracting extremely large numbers, you must follow these rules. The first number has to be between 1. 0 and 9. 9 The exponent must match the number of spaces you have moved the decimal to make the number work If the original number is smaller the exponent is NEGATIVE If the original number is larger the exponent is POSITIVE Ex: 2, 500 = 2. 5 x 103 Ex: 0. 0025 = 2. 5 x 10 -3

Contraction Practice 4, 000 = 0. 03 = 5, 268 = 0. 000123 = 12 =

Earth Science Measurements Mass: The amount of matter (atoms and molecules) in an object Expressed in grams Weight: the pull of gravity on an object toward the center of the earth

Mass vs. Weight Do people weight the same on the moon as they do on earth? Why? Do you have the same mass?

Mass vs Weight You become “weightless” in space because the force of gravity is so low. Similar to magnets Your mass will be the same on earth, on the moon, on Jupiter, even on Uranus.

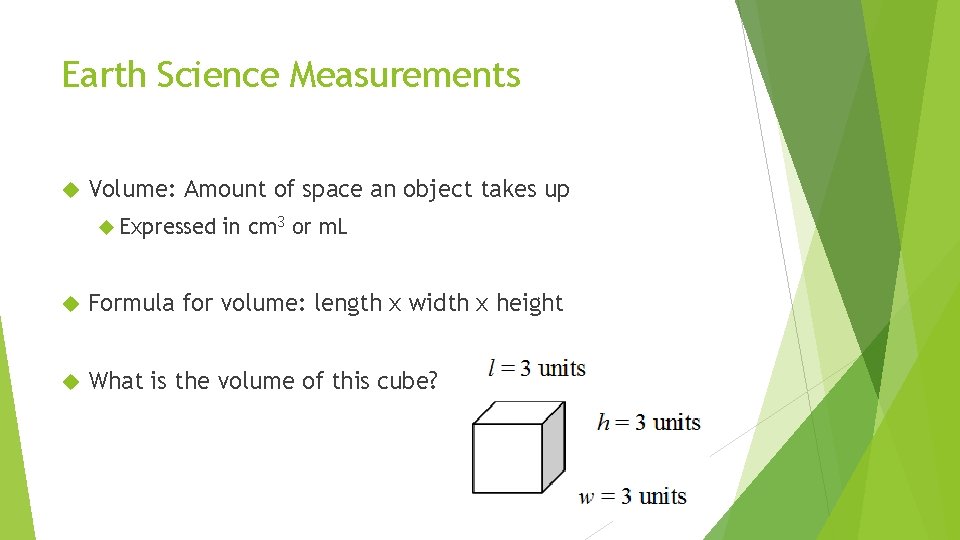
Earth Science Measurements Volume: Amount of space an object takes up Expressed in cm 3 or m. L Formula for volume: length x width x height What is the volume of this cube?

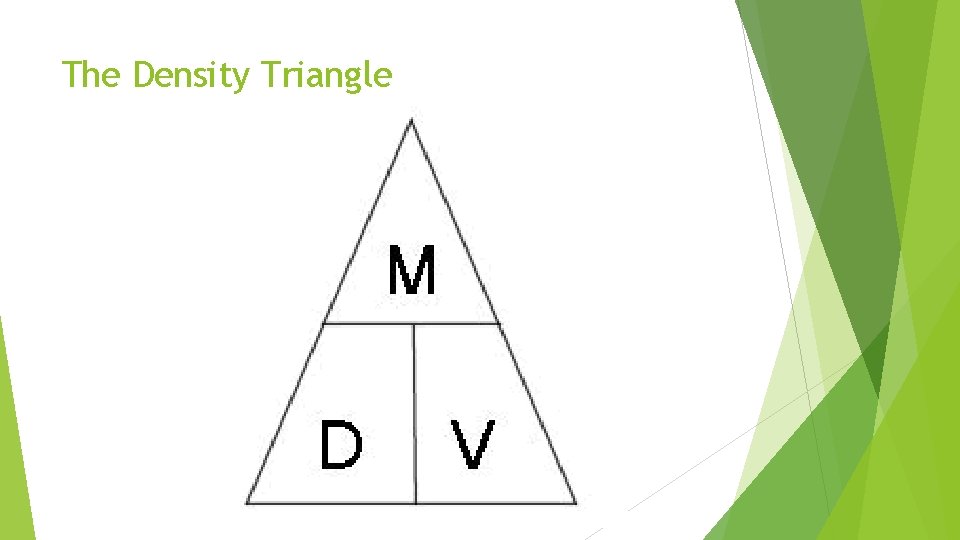
Earth Science Measurements Density: Amount of matter in an amount of space. How compacted something is. Expressed in grams per cubic centimeter (g/cm 3) Formula for density = mass divided by volume (D = M/V) The density of water is 1 g/cm 3 at about 4 degrees Celcius

The Density Triangle

Density How can we make this room more dense? How can we make this room less dense?



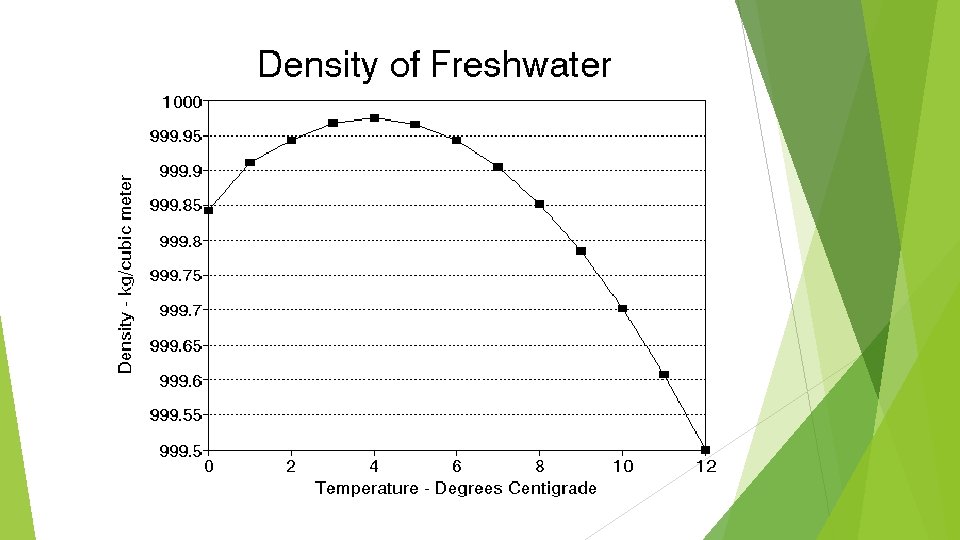
Density Usually, the phase of matter (solid, liquid, gas) is determined by how closely packed the atoms are. Most of earths materials are most dense as a solid and least dense as a gas, with liquid in the middle. Then why does ice float?

Density of Water is special in the case that it expands as it freezes making it less dense. Water is most dense as a liquid at 4 degrees Celsius (1 g/cm 3)
