Unit 1 Matter and Energy Section 2 Measurements
- Slides: 16

Unit 1: Matter and Energy Section 2: Measurements and Calculations

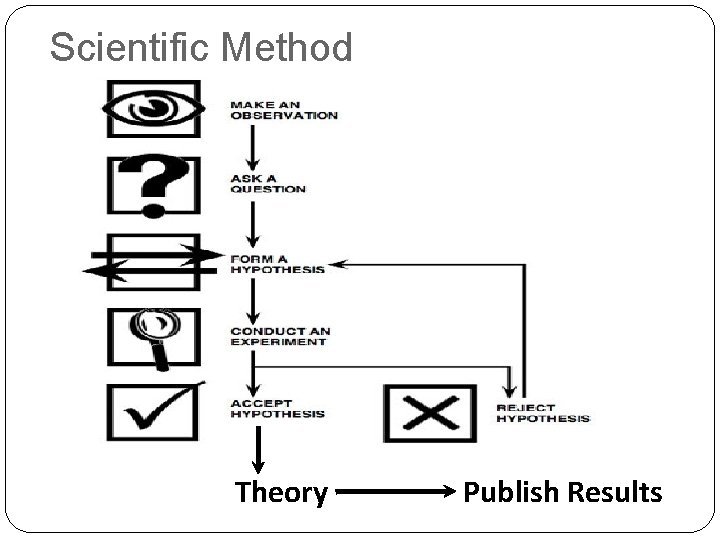
Scientific Method Theory Publish Results

Units of Measure Qualitative Quantitative Descriptive (Non-numeric) Measurement (Numeric) The fact that the sky is blue A sample of copper ore has a mass of 25. 7 grams

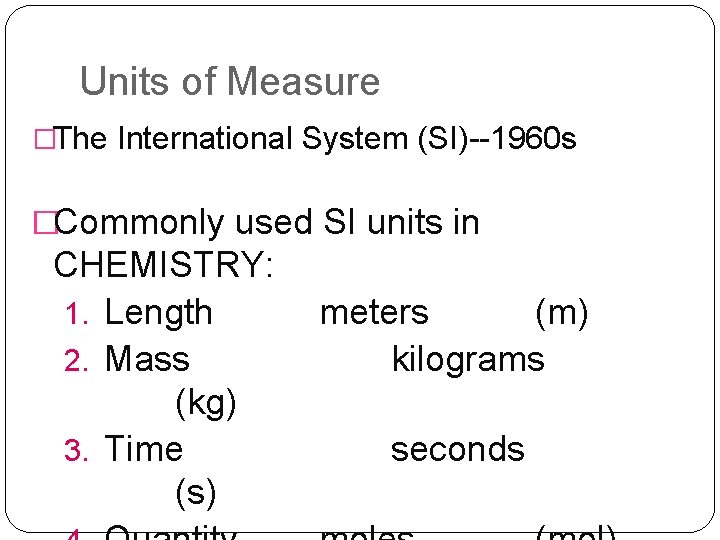
Units of Measure �The International System (SI)--1960 s �Commonly used SI units in CHEMISTRY: 1. Length 2. Mass (kg) 3. Time (s) meters (m) kilograms seconds

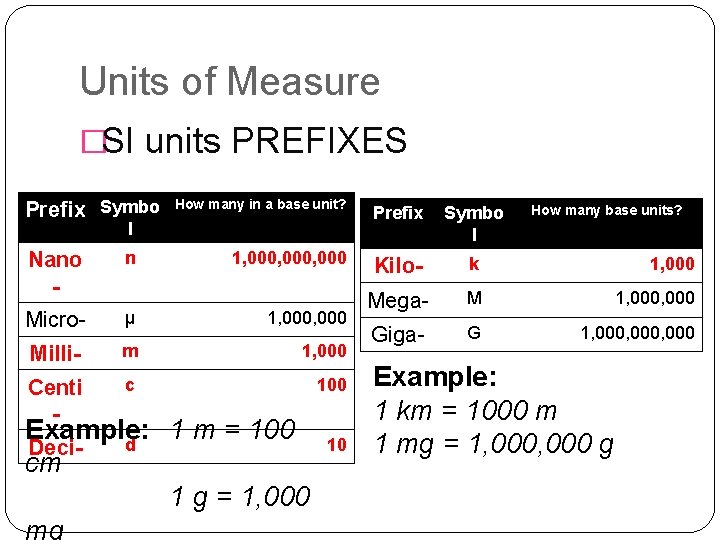
Units of Measure �SI units PREFIXES Prefix Symbo How many in a base unit? Prefix Symbo l 1, 000, 000 Kilo- k 1, 000 Mega- M 1, 000 Giga- G 1, 000, 000 l Nano - n Micro- μ 1, 000 Milli- m 1, 000 Centi - c 100 Example: 1 m = 100 d Decicm 1 g = 1, 000 mg 10 How many base units? Example: 1 km = 1000 m 1 mg = 1, 000 g

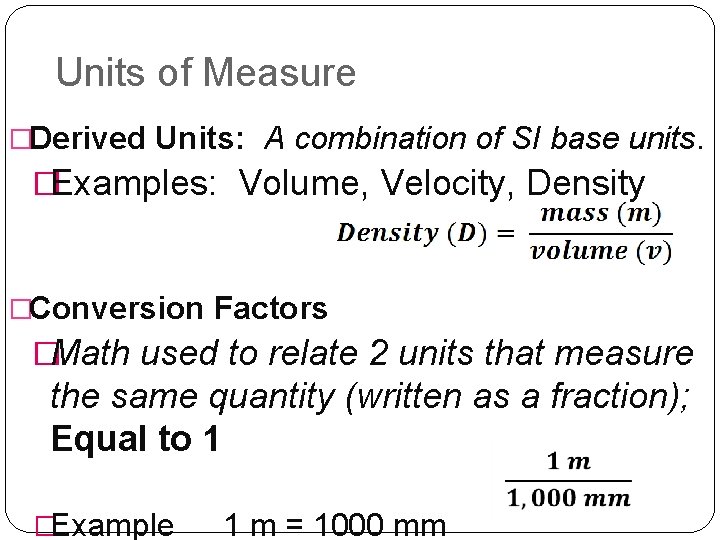
Units of Measure �Derived Units: A combination of SI base units. �Examples: Volume, Velocity, Density �Conversion Factors �Math used to relate 2 units that measure the same quantity (written as a fraction); Equal to 1 �Example 1 m = 1000 mm

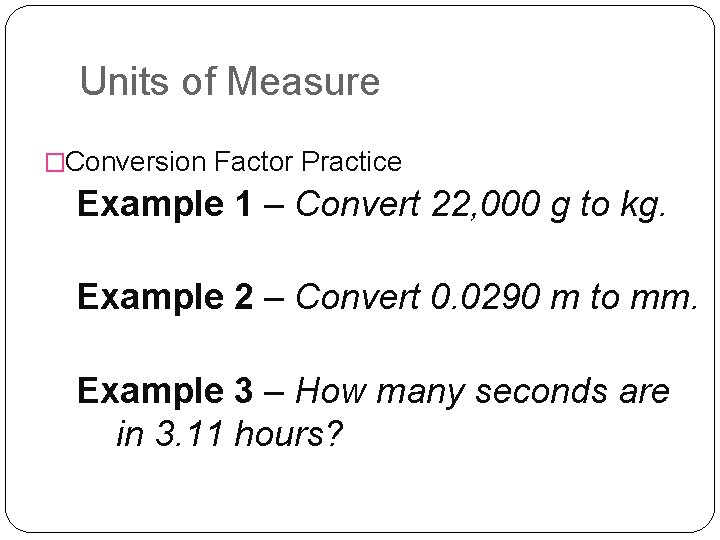
Units of Measure �Conversion Factor Practice Example 1 – Convert 22, 000 g to kg. Example 2 – Convert 0. 0290 m to mm. Example 3 – How many seconds are in 3. 11 hours?

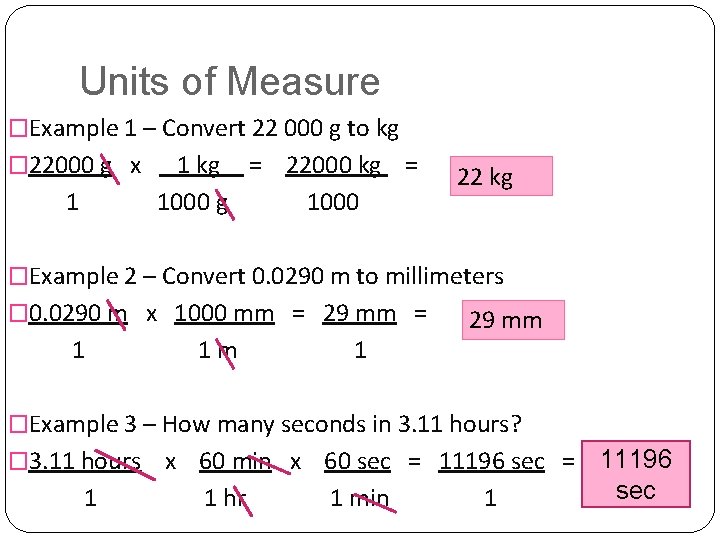
Units of Measure �Example 1 – Convert 22 000 g to kg � 22000 g x 1 1 kg = 22000 kg = 1000 g 1000 22 kg �Example 2 – Convert 0. 0290 m to millimeters � 0. 0290 m x 1000 mm = 29 mm = 1 1 m 1 29 mm �Example 3 – How many seconds in 3. 11 hours? � 3. 11 hours 1 x 60 min x 60 sec = 11196 sec 1 hr 1 min 1

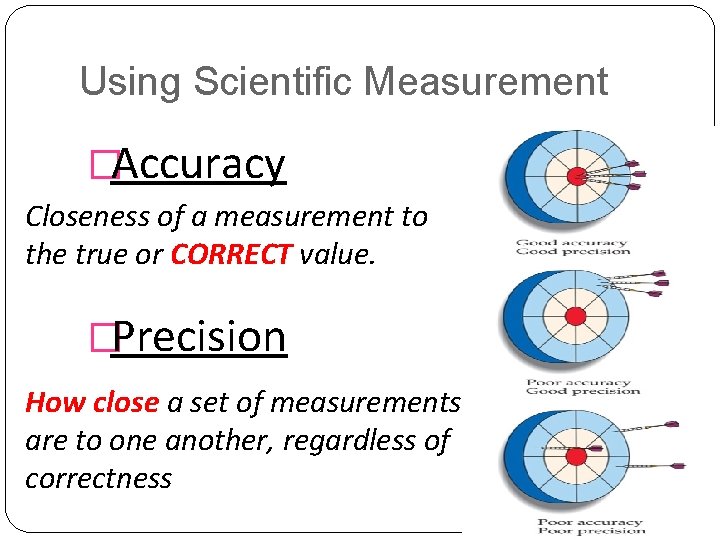
Using Scientific Measurement �Accuracy Closeness of a measurement to the true or CORRECT value. �Precision How close a set of measurements are to one another, regardless of correctness

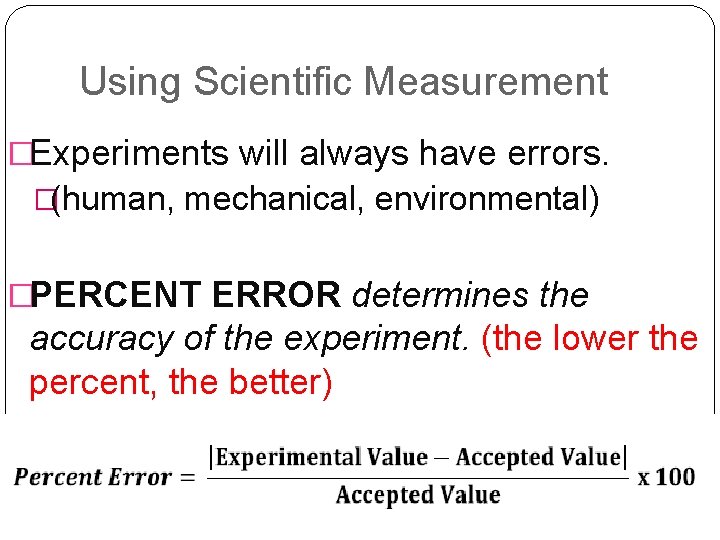
Using Scientific Measurement �Experiments will always have errors. �(human, mechanical, environmental) �PERCENT ERROR determines the accuracy of the experiment. (the lower the percent, the better)

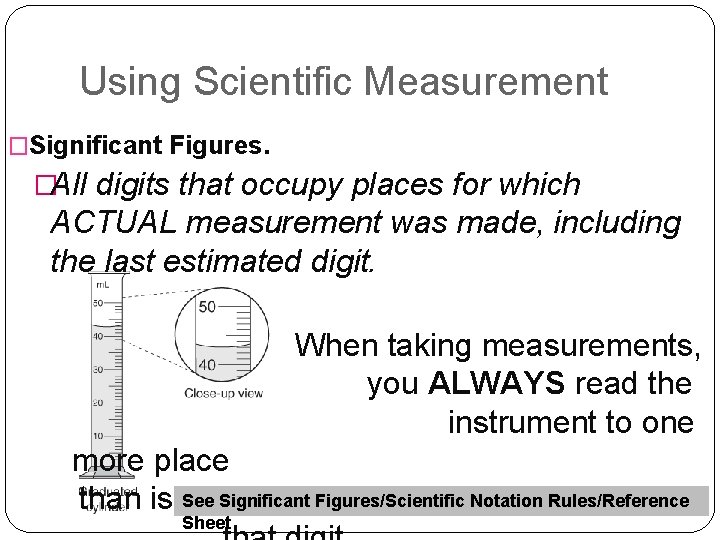
Using Scientific Measurement �Significant Figures. �All digits that occupy places for which ACTUAL measurement was made, including the last estimated digit. When taking measurements, you ALWAYS read the instrument to one more place Significant Figures/Scientific Notation Rules/Reference than is See marked. You estimate Sheet

Significant Figures Practice 1) 30 504 � 5 sig. figs. 2) 32. 001 20 � 7 sig. figs. 3) 0. 000 123 0 � 4 sig figs. 4) 560 000 � 2 sig. figs. 5) 2 000. 003 � 7 sig. figs.

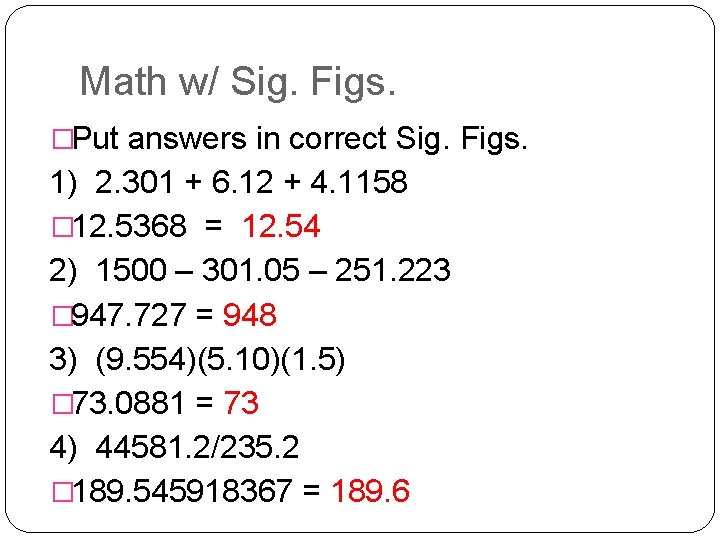
Math w/ Sig. Figs. �Put answers in correct Sig. Figs. 1) 2. 301 + 6. 12 + 4. 1158 � 12. 5368 = 12. 54 2) 1500 – 301. 05 – 251. 223 � 947. 727 = 948 3) (9. 554)(5. 10)(1. 5) � 73. 0881 = 73 4) 44581. 2/235. 2 � 189. 545918367 = 189. 6

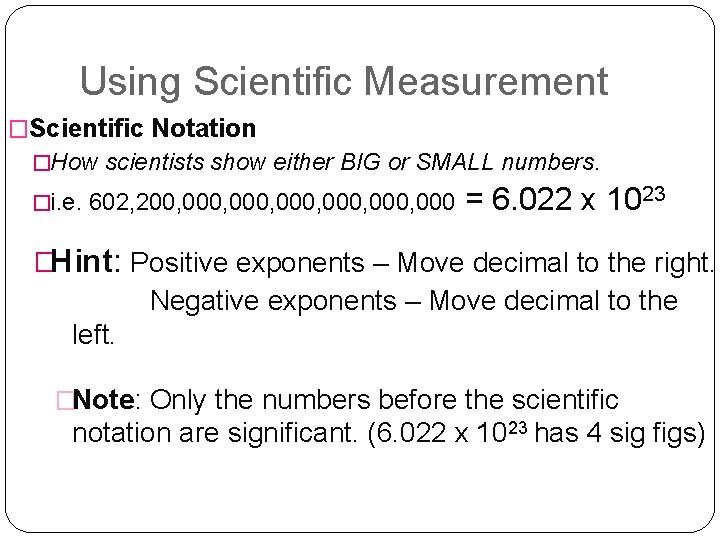
Using Scientific Measurement �Scientific Notation �How scientists show either BIG or SMALL numbers. �i. e. 602, 200, 000, 000 = 6. 022 x 1023 �Hint: Positive exponents – Move decimal to the right. Negative exponents – Move decimal to the left. �Note: Only the numbers before the scientific notation are significant. (6. 022 x 1023 has 4 sig figs)

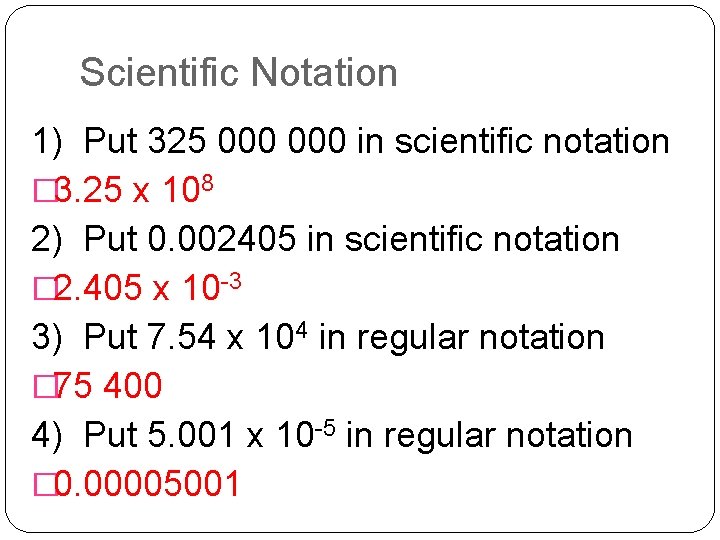
Scientific Notation 1) Put 325 000 in scientific notation � 3. 25 x 108 2) Put 0. 002405 in scientific notation � 2. 405 x 10 -3 3) Put 7. 54 x 104 in regular notation � 75 400 4) Put 5. 001 x 10 -5 in regular notation � 0. 00005001

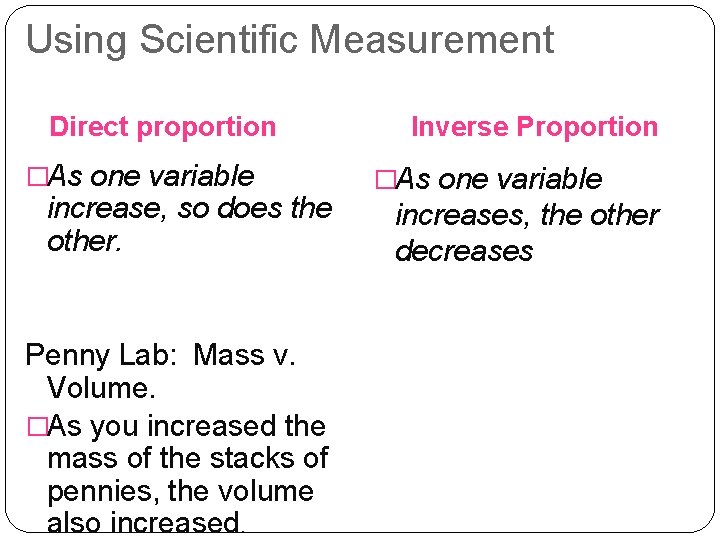
Using Scientific Measurement Direct proportion �As one variable increase, so does the other. Penny Lab: Mass v. Volume. �As you increased the mass of the stacks of pennies, the volume also increased. Inverse Proportion �As one variable increases, the other decreases