Unit 1 Matter And Energy Chemistry In this























- Slides: 58

Unit 1: Matter And Energy

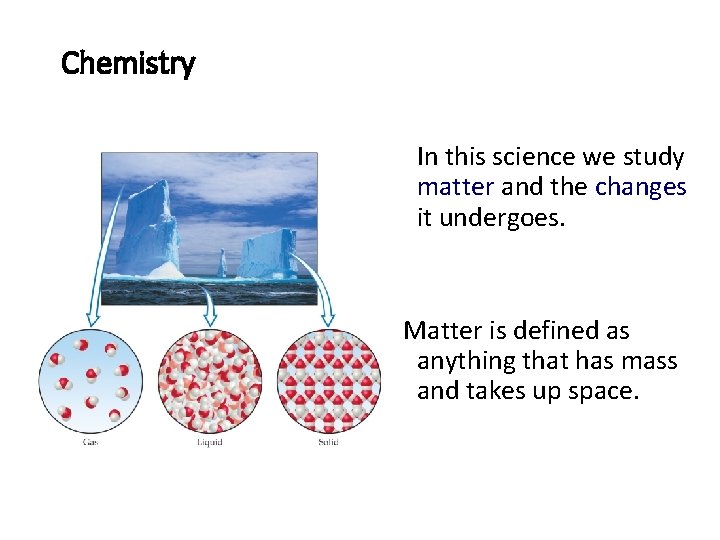
Chemistry In this science we study matter and the changes it undergoes. Matter is defined as anything that has mass and takes up space.

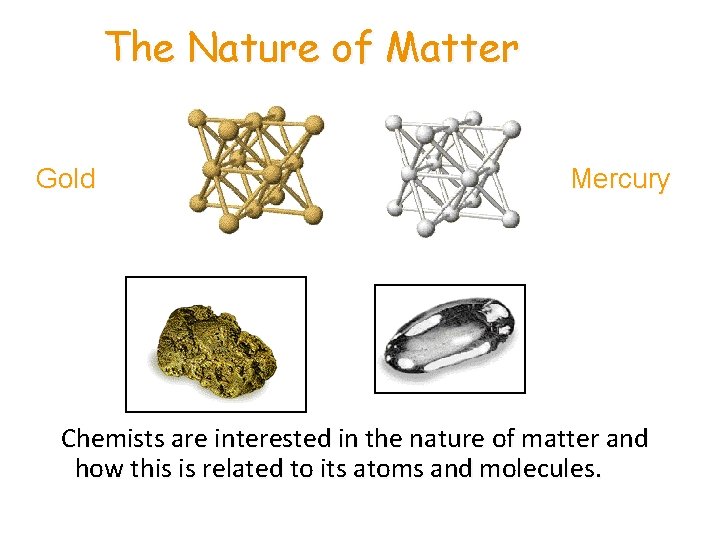
The Nature of Matter Gold Mercury Chemists are interested in the nature of matter and how this is related to its atoms and molecules.

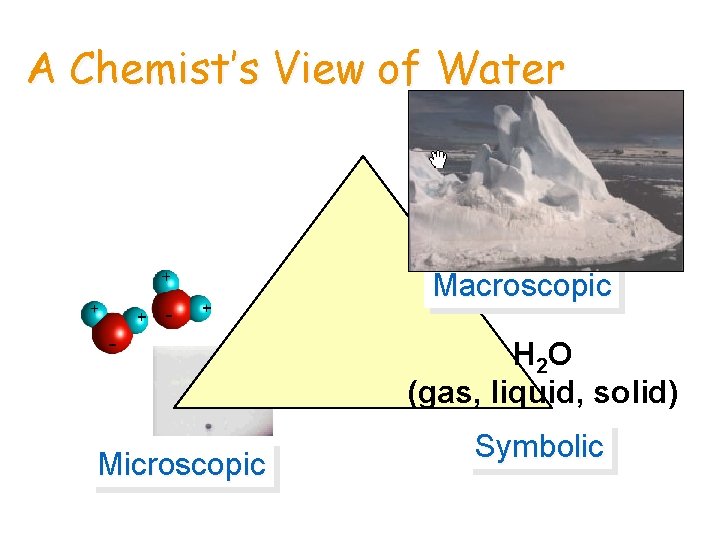
Chemistry & Matter • We can explore the MACROSCOPIC world — what we can see — • to understand the MICROSCOPIC or SUBATOMIC worlds we cannot see. • We write SYMBOLS to describe these worlds.

A Chemist’s View of Water Macroscopic H 2 O (gas, liquid, solid) Microscopic Symbolic

Kinetic Molecular Theory

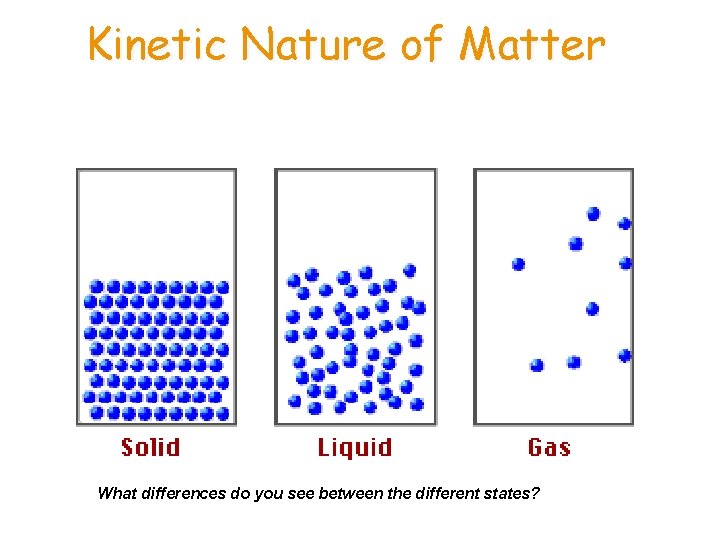
Kinetic Nature of Matter What differences do you see between the different states?

STATES OF MATTER • _______ — have rigid, fixed shape and volume. External shape can reflect the atomic and molecular arrangement. • Little space between atoms, little energy • Most dense state (one exception) • _______ — have no definite shape but have definite volume • Some space between molecules, medium energy • Have a medium density • _______ — expand to fill their container. • Indefinite shape and indefinite volume • A lot of space between molecules, High energy • Have very low density

OTHER STATES OF MATTER • PLASMA — an electrically charged gas; Example: the sun or any other star • Aqueous — a substance dissolved in water. Example: an aqueous salt is a salt dissolved in water

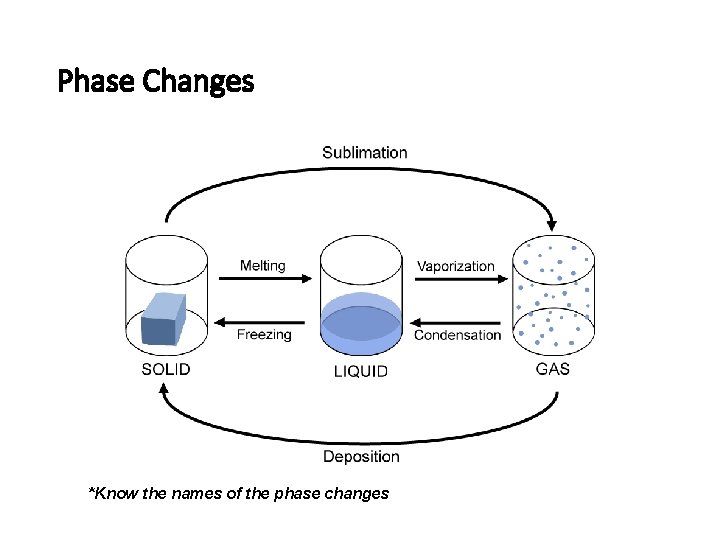
Phase Changes *Know the names of the phase changes

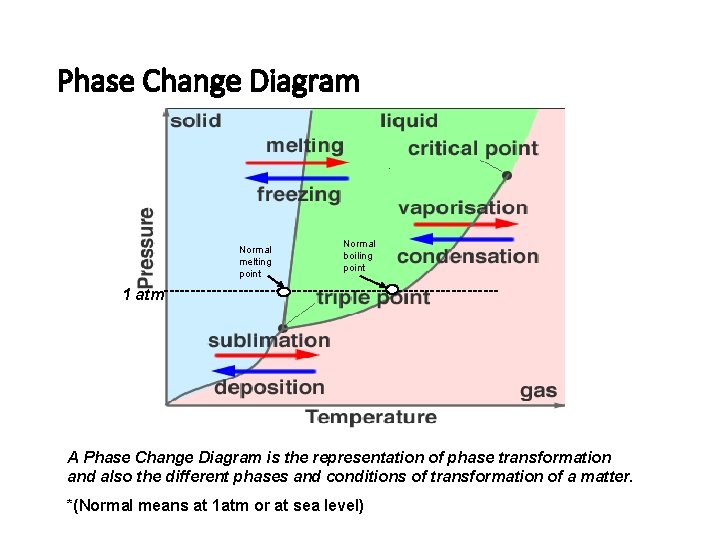
Phase Change Diagram Normal melting point Normal boiling point 1 atm-------------------------------- A Phase Change Diagram is the representation of phase transformation and also the different phases and conditions of transformation of a matter. *(Normal means at 1 atm or at sea level)

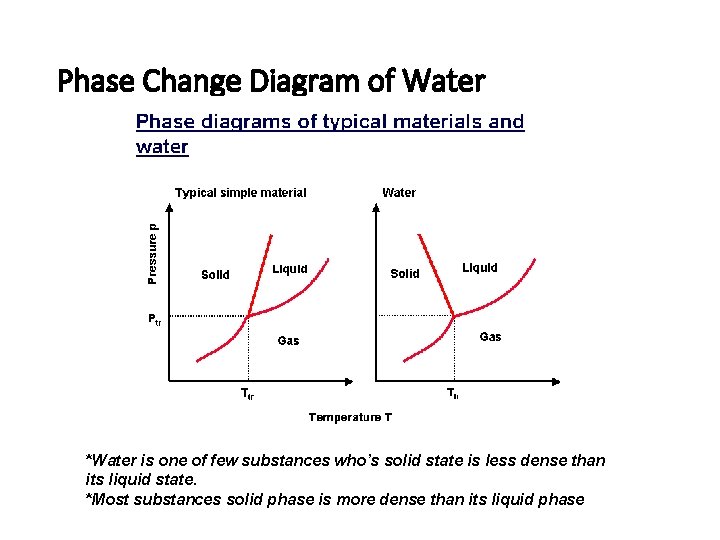
Phase Change Diagram of Water *Water is one of few substances who’s solid state is less dense than its liquid state. *Most substances solid phase is more dense than its liquid phase

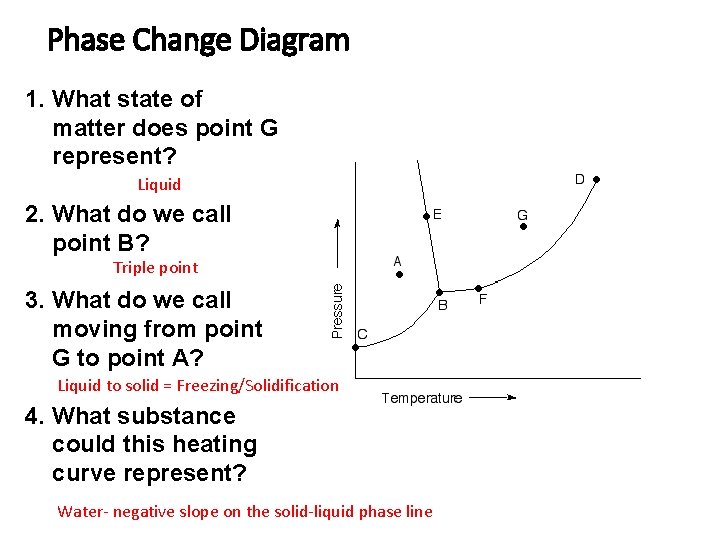
Phase Change Diagram 1. What state of matter does point G represent? Liquid 2. What do we call point B? Triple point 3. What do we call moving from point G to point A? Liquid to solid = Freezing/Solidification 4. What substance could this heating curve represent? Water- negative slope on the solid-liquid phase line

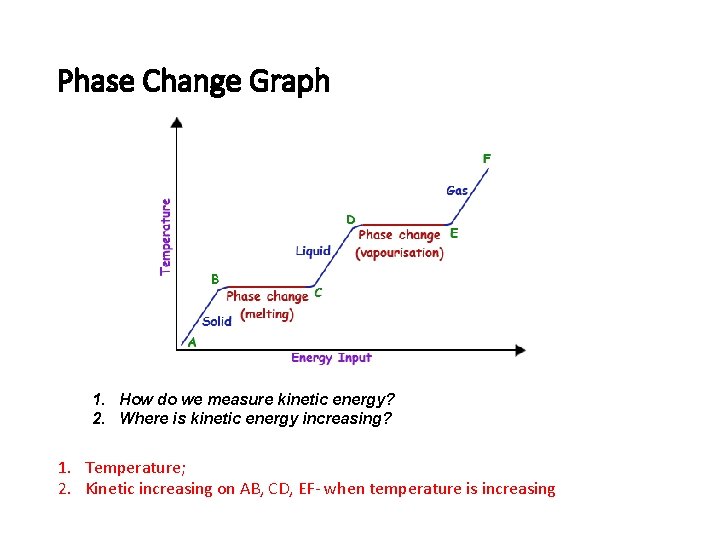
Phase Change Graph 1. How do we measure kinetic energy? 2. Where is kinetic energy increasing? 1. Temperature; 2. Kinetic increasing on AB, CD, EF- when temperature is increasing

Click the link for phase change • Phase change demonstration

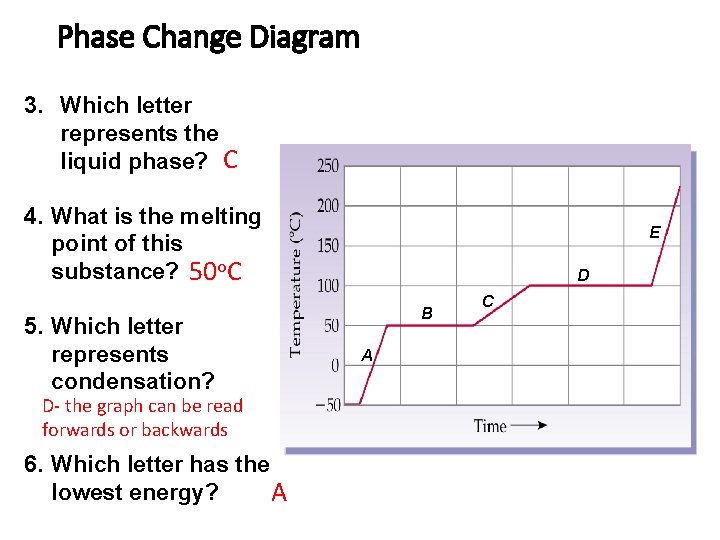
Phase Change Diagram 3. Which letter represents the liquid phase? C 4. What is the melting point of this substance? 50 o. C 5. Which letter represents condensation? D- the graph can be read forwards or backwards 6. Which letter has the lowest energy? A E D B A C

Types of Properties • Intensive Properties… • Are independent of the amount of the substance that is present. • Density, boiling point, color, etc. • Extensive Properties… • Depend upon the amount of the substance present. • Mass, volume, energy, etc.

Types of Properties • Physical Properties… • Can be observed without changing a substance into another substance. • Chemical Properties… • Can only be observed when a substance is changed into another substance/ when a chemical reaction occurs.

Physical Properties What are some physical properties? • Color • Melting and boiling point • Odor • Shape • Density • Phase of matter

Chemical Properties • Examples of Chemical Properties: • Heat of combustion • Flammability • Toxicity • Reactivity • Rusting

Physical Changes • can be observed without changing the identity of the substance Some physical changes would be • Crushing • Cutting • Boiling of a liquid • Melting of a solid • Dissolving a solid in a liquid to give a homogeneous mixture — a SOLUTION. • Phase changes are always physical

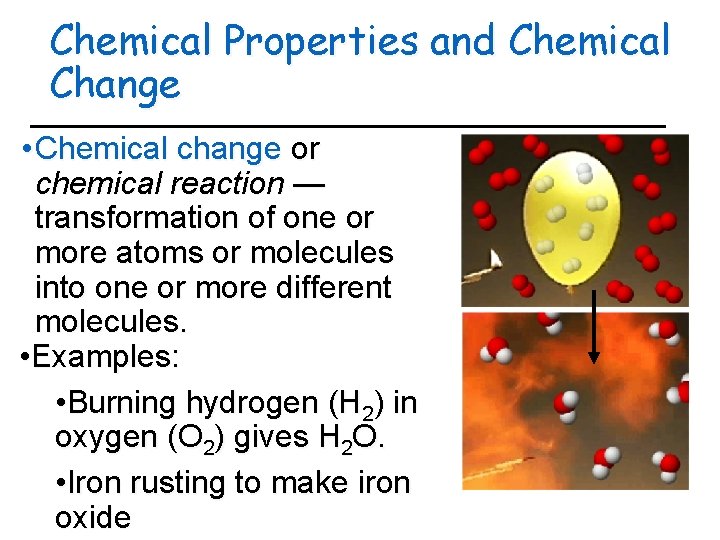
Chemical Properties and Chemical Change • Chemical change or chemical reaction — transformation of one or more atoms or molecules into one or more different molecules. • Examples: • Burning hydrogen (H 2) in oxygen (O 2) gives H 2 O. • Iron rusting to make iron oxide

Sure Indicators of a Chemical Change • Heat • Light • Effervescence- bubbling/ Gas Produced (not from boiling!) • Odor produced • Precipitate – a solid formed by mixing two liquids together • Looks like a solid or looks cloudy • Some times color

Physical vs. Chemical Properties • Examples: • melting point physical • flammable chemical • density physical • magnetic physical • tarnishes in air chemical

Physical vs. Chemical Changes • Examples: • rusting iron chemical • dissolving in water physical • burning a log chemical • melting ice physical • grinding spices physical

Matter Flowchart MATTER yes MIXTURE yes Is the composition uniform? Homogeneous Mixture (solution) no Can it be physically separated? PURE SUBSTANCE no Heterogeneous Mixture yes Can it be chemically decomposed? Compound no Element

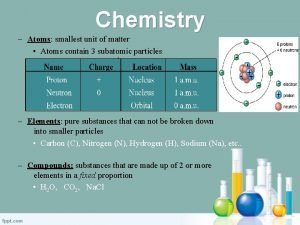
Elements and Compounds • Atoms are the building blocks of matter. • Each element is made of the same kind of atom. • Represented by elemental symbol • Example: Au or O • A compound is made of two or more different kinds of elements that are chemically bonded. • Represented by a chemical formula (example H 2 O) • Always in definite proportions • A pure substance refers to an element or a compound

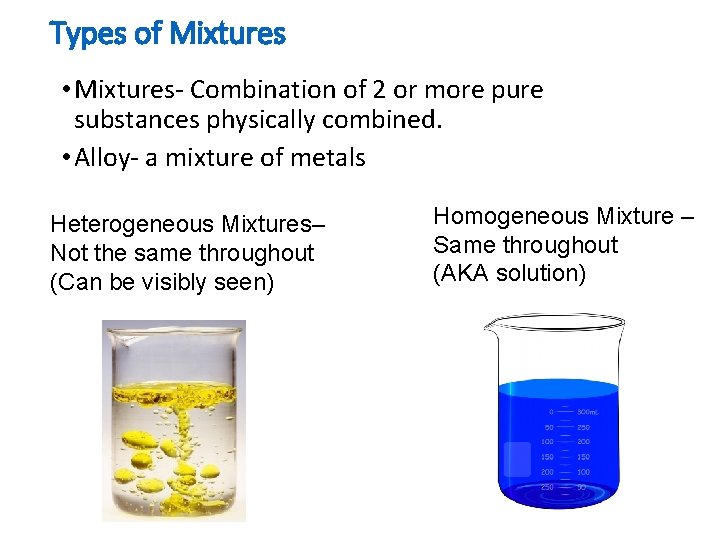
Types of Mixtures • Mixtures- Combination of 2 or more pure substances physically combined. • Alloy- a mixture of metals Heterogeneous Mixtures– Not the same throughout (Can be visibly seen) Homogeneous Mixture – Same throughout (AKA solution)

Examples • Label the following as an element, compound, solution, alloy, or heterogeneous mixture Solution 1. Lemonade 2. Sodium Chloride Compound 3. Gold Element 4. Steal Alloy 5. Pizza Heterogeneous mixture 6. Sulfur Element 7. Oil and water Mixture

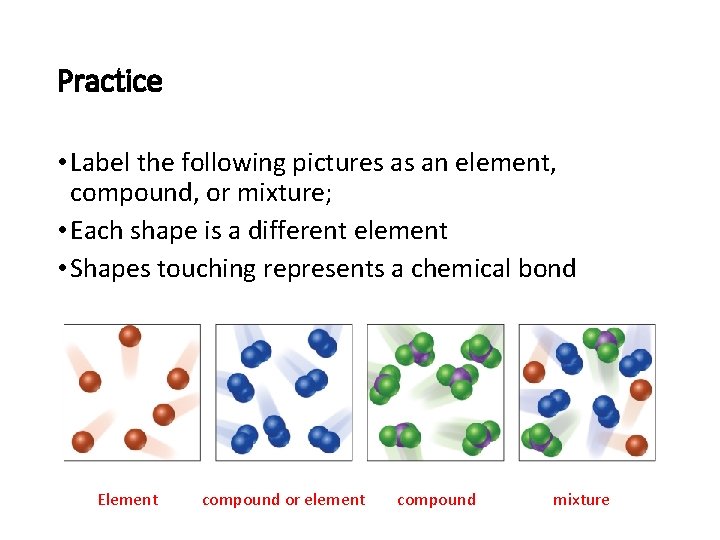
Practice • Label the following pictures as an element, compound, or mixture; • Each shape is a different element • Shapes touching represents a chemical bond Element compound or element compound mixture

Physical and Chemical Separation • Physical separation is separation without chemically changing the composition of a substance • Only mixtures and solutions can be physically separated • Example: separating a solution of salt water by boiling off the water • The salt and water are not chemically changed they are just physically separated from each other • Chemical separation is the separation of a compound by chemically changing the composition of a substance • Involves breaking of chemical bonds • Only compounds can be chemically separated • Example: separating a water molecule into hydrogen and oxygen molecules using electrolysis • The chemical bonds within the water molecule are broken • An atom cannot be chemically separated nor physically separated

Physical Separation of Mixtures • Mixtures can be physically separated using different techniques • Pure substances can NOT be physically separated • Physical separation techniques use differences in properties to separate the substances

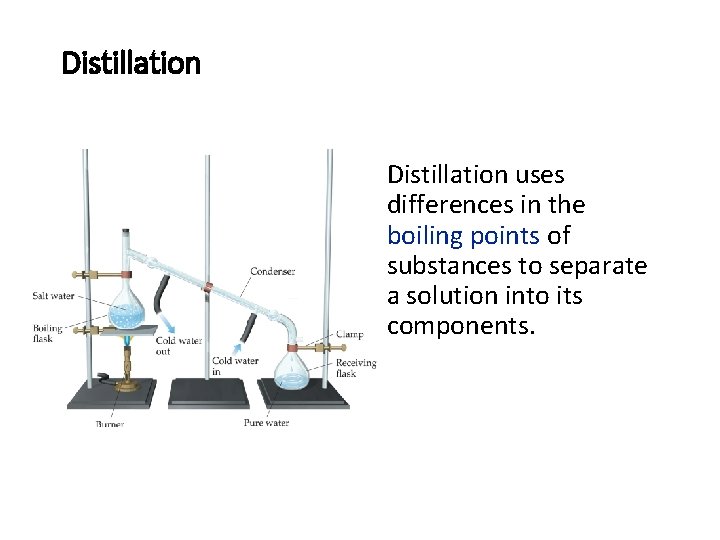
Distillation uses differences in the boiling points of substances to separate a solution into its components.

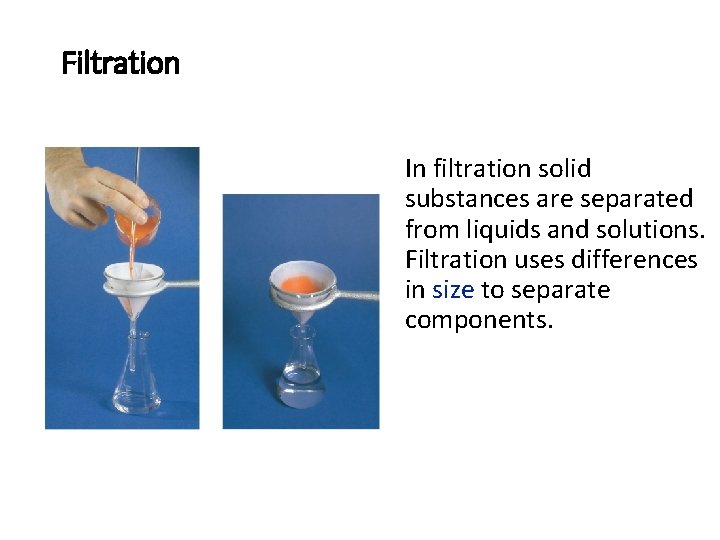
Filtration In filtration solid substances are separated from liquids and solutions. Filtration uses differences in size to separate components.

Chromatography This technique separates substances on the basis of differences in properties including size, solubility (ability to dissolve), boiling point, and/or mass.

Scientific Method 1. Observation 2. Question 3. Hypothesis 4. Method 5. Results 6. Conclusion

Energy • Energy is the ability to do work or produce heat • Mostly covered in physics • Two types • Kinetic – energy in motion • Potential - stored energy • All chemical reactions require energy to get started • This amount of energy required is called the activation energy (Ea)

Energy • Temperature is a measure of average kinetic energy • Energy can be measured in Joules, Calories, Kilocalories (we use Joules) • Law of Conservation of Energy: energy can be converted from one form to another but can be neither created nor destroyed • When one object or reaction loses energy, that same amount of energy is gained by something else • q is used to represent heat energy • A reaction in which heat is lost or released is considered exothermic • Feels hot, q is negative • A reaction in which heat is gained or absorbed is considered endothermic • Feels cold, q is positive

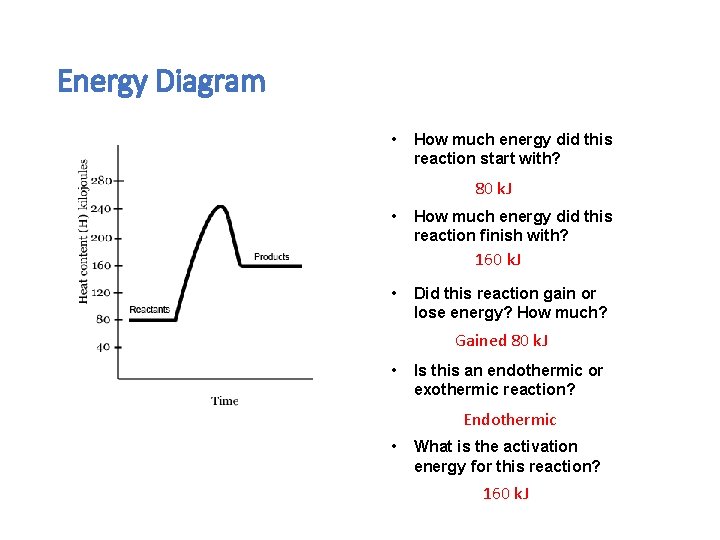
Energy Diagram • How much energy did this reaction start with? 80 k. J • How much energy did this reaction finish with? 160 k. J • Did this reaction gain or lose energy? How much? Gained 80 k. J • Is this an endothermic or exothermic reaction? Endothermic • What is the activation energy for this reaction? 160 k. J

Energy Diagram (k. J) • How much energy did this reaction start with? 150 k. J • How much energy did this reaction finish with? 50 k. J • Did this reaction gain or lose energy? How much? Lost 100 k. J • Is this an endothermic or exothermic reaction? Exothermic • What is the Ea for this reaction? 50 k. J

Types of Observations and Measurements § A qualitative measurement is something that cannot be measured in numbers. § Usually referred to as an observation § Ex: odor, texture, color § Quantitative measurements are those which involve the collection of numbers. § Ex: temperature, mass, volume § In chemistry SI units are used (International System of Units) — based on the metric system § Ex: Grams, meters, liters § As opposed to U. S customary units § Ex: Pounds, feet, gallons

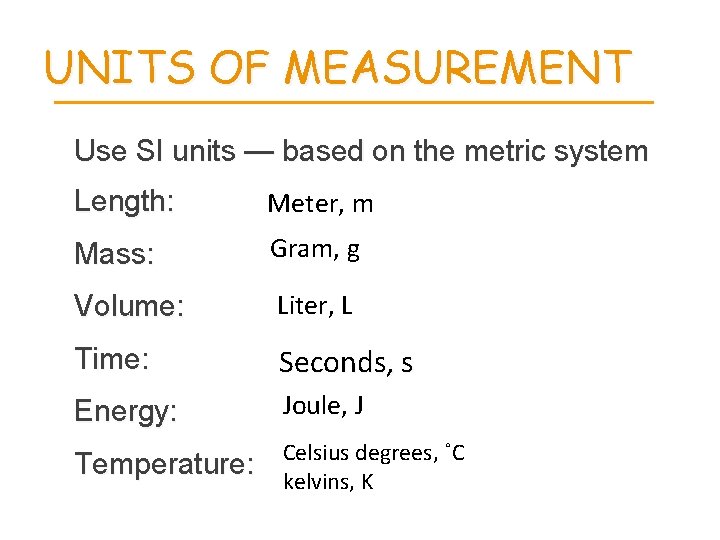
UNITS OF MEASUREMENT Use SI units — based on the metric system Length: Meter, m Mass: Gram, g Volume: Liter, L Time: Seconds, s Energy: Joule, J Temperature: Celsius degrees, ˚C kelvins, K

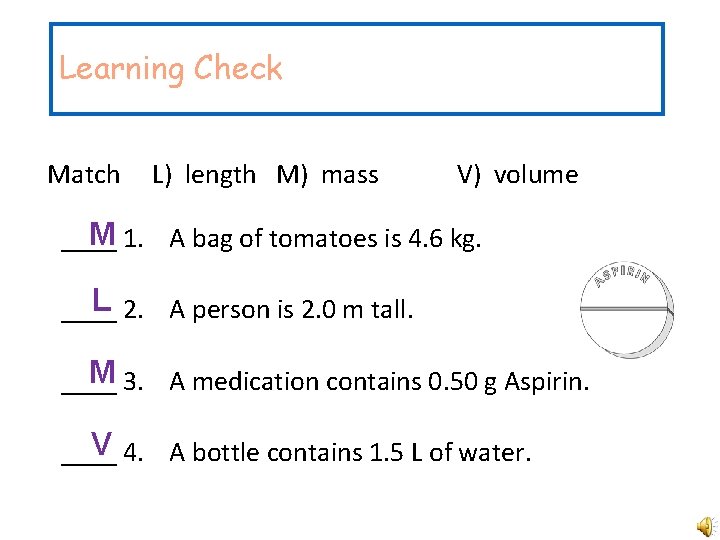
Learning Check Match L) length M) mass V) volume M 1. A bag of tomatoes is 4. 6 kg. ____ L 2. A person is 2. 0 m tall. ____ M 3. A medication contains 0. 50 g Aspirin. ____ V 4. A bottle contains 1. 5 L of water. ____

Some Tools for Measurement Which tool(s) would you use to measure: A. temperature B. volume C. time D. mass There is a list of commonly used lab equipment in the Unit 1 Resources. You need to be able to identify the lab equipment and know what it is used for.

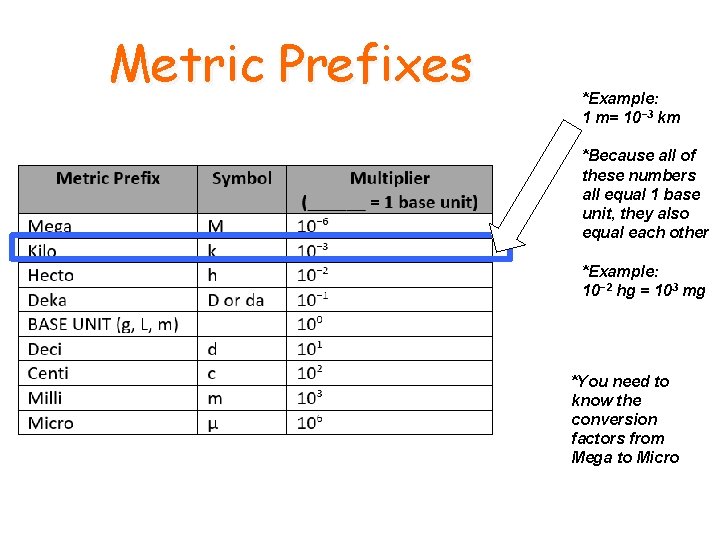
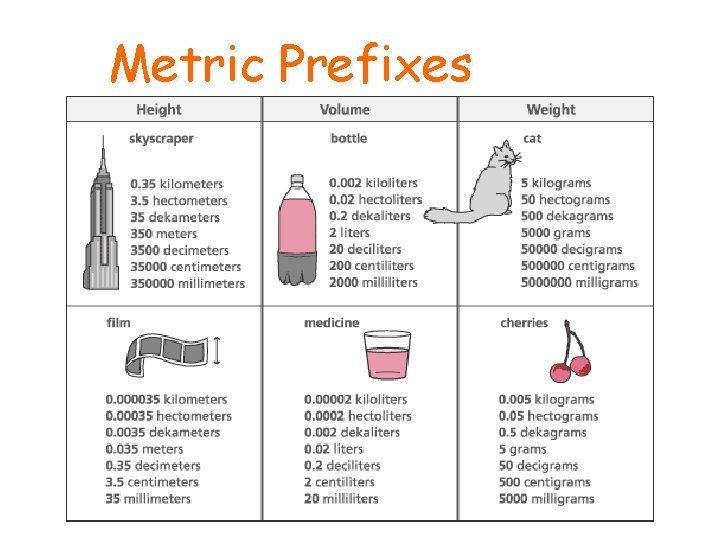
Metric Units • The base units for the metric system are • Grams (g) • Liters (L) • Meters (m) • Prefixes are used with these base units to represent a factor of the base unit

Metric Prefixes *Example: 1 m= 10− 3 km *Because all of these numbers all equal 1 base unit, they also equal each other *Example: 10− 2 hg = 103 mg *You need to know the conversion factors from Mega to Micro

Metric Prefixes

Metric Prefixes • Kilo (k)- means 1000 of that whole unit • Hecto (H)- means 100 of that whole unit • Deca (D)- means 10 of that whole unit • Deci (d)- means 1/10 of a unit • Centi (c)- means 1/100 of a unit • Milli (m)- means 1/1000 of a unit

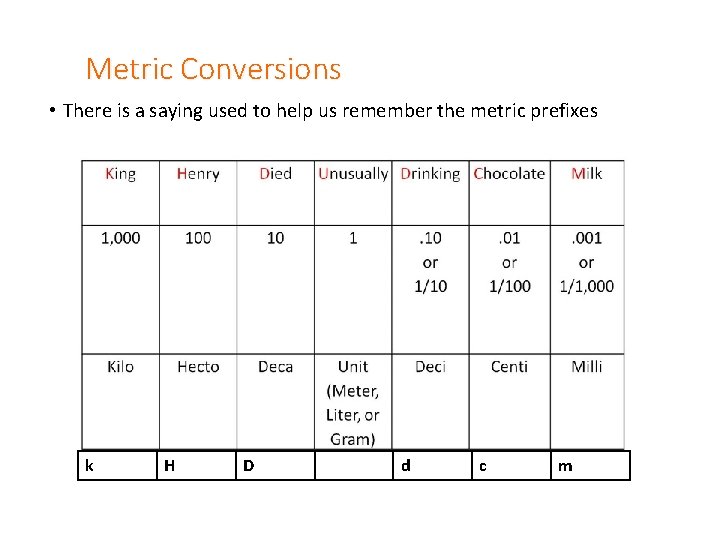
Metric Conversions • There is a saying used to help us remember the metric prefixes k H D d c m

Metric Conversions • Convert 3. 400 kiloliters to decaliters To convert using metrics: 1. Determine which prefix you are starting with 2. Determine which prefix you are converting to 3. Count how many spaces you move when you go from step 1 to step 2 4. Move the decimal over that many spaces in the same direction Move the decimal over 2 Start spaces to the right 3. 400 k. L = 340. 0 DL Finish

Learning Check Select the unit you would use to measure 1. Your height a) millimeters b) meters c) kilometers 2. Your mass a) milligrams b) grams c) kilograms 3. The distance between two cities a) millimeters b) meters c) kilometers 4. The width of an artery a) millimeters b) meters c) kilometers

Learning Check 5. 1000 mm = ___km 4. 0. 00125 kg = ___ cg 5. 0. 56 L = ___c. L 6. 0. 099 m = ___ dm 0. 001 km 125 cg 56 c. L 0. 99 dm

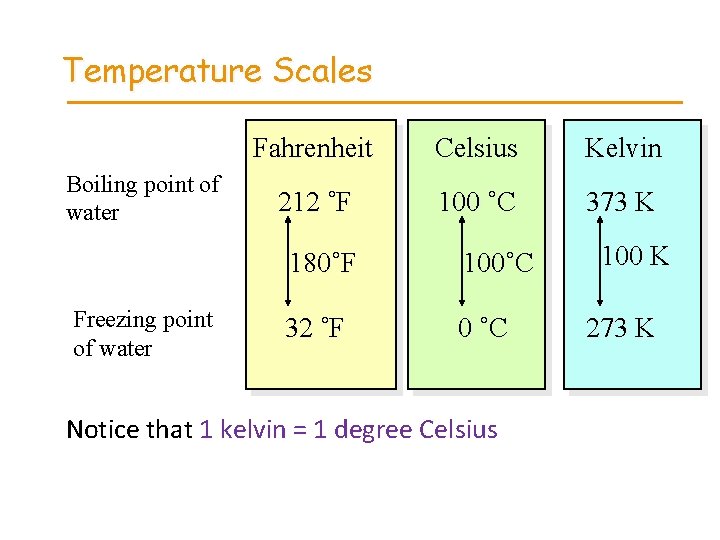
Temperature Scales • Fahrenheit (o. F) • Celsius (o. C) • Kelvin (K) Anders Celsius 1701 -1744 Lord Kelvin (William Thomson) 1824 -1907

Temperature Scales Boiling point of water Freezing point of water Fahrenheit Celsius Kelvin 212 ˚F 100 ˚C 373 K 180˚F 100˚C 32 ˚F 0 ˚C Notice that 1 kelvin = 1 degree Celsius 100 K 273 K

Accuracy and Precision Three targets with three arrows each to shoot. How do they compare? Both accurate and precise Precise but not accurate Neither accurate nor precise Can you define accuracy and precision? Accuracy refers to the correctness and precision refers to consistency of measurements

Density • Density is mass per unit volume • D= m/V • Density is measured in units of g/cm 3 or g/m. L • Note: 1 m. L = 1 cm 3 • Density is a physical property • The density of water is 1. 00 g/m. L • When calculating density, round to 2 decimal places • How would you determine the density of a metal experimentally? • Measure mass using a scale and • Measure volume using • A meter stick; measuring length, width, and height • Water displacement; Measure an initial volume of water, place the object in the water, measure the final volume of both water and object, subtract the final and initial volumes to get volume of the object.

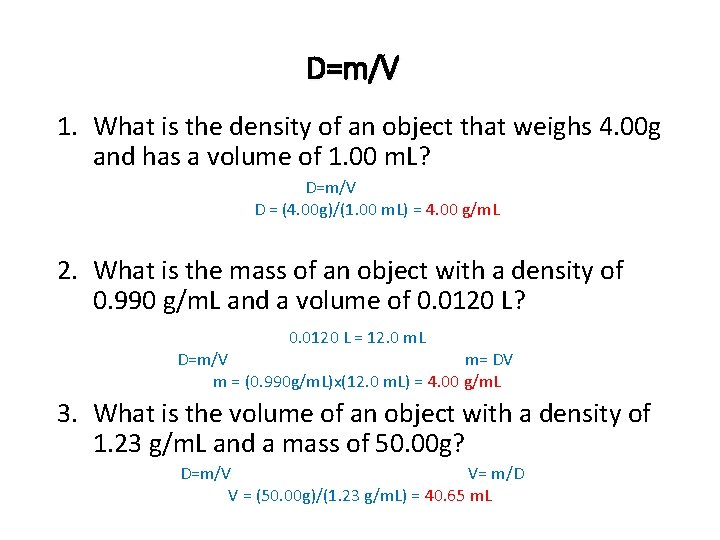
D=m/V 1. What is the density of an object that weighs 4. 00 g and has a volume of 1. 00 m. L? D=m/V D = (4. 00 g)/(1. 00 m. L) = 4. 00 g/m. L 2. What is the mass of an object with a density of 0. 990 g/m. L and a volume of 0. 0120 L? 0. 0120 L = 12. 0 m. L D=m/V m= DV m = (0. 990 g/m. L)x(12. 0 m. L) = 4. 00 g/m. L 3. What is the volume of an object with a density of 1. 23 g/m. L and a mass of 50. 00 g? D=m/V V= m/D V = (50. 00 g)/(1. 23 g/m. L) = 40. 65 m. L

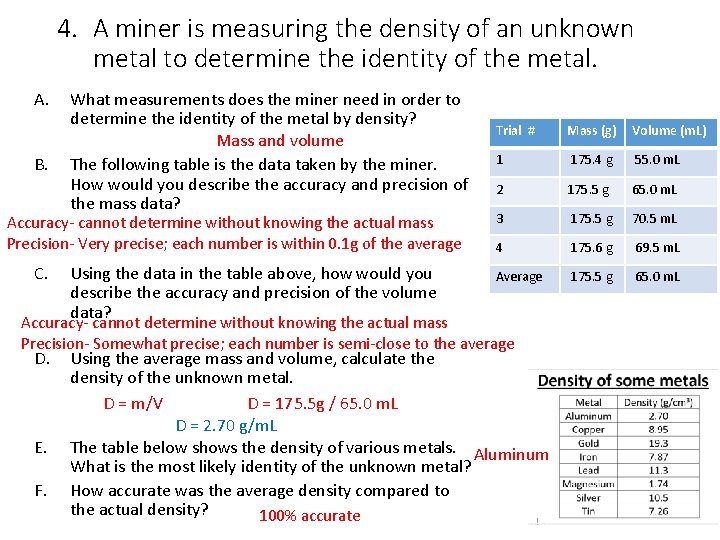
4. A miner is measuring the density of an unknown metal to determine the identity of the metal. A. B. What measurements does the miner need in order to determine the identity of the metal by density? Mass and volume The following table is the data taken by the miner. How would you describe the accuracy and precision of the mass data? Accuracy- cannot determine without knowing the actual mass Precision- Very precise; each number is within 0. 1 g of the average C. Using the data in the table above, how would you describe the accuracy and precision of the volume data? Trial # Mass (g) Volume (m. L) 1 175. 4 g 55. 0 m. L 2 175. 5 g 65. 0 m. L 3 175. 5 g 70. 5 m. L 4 175. 6 g 69. 5 m. L Average 175. 5 g 65. 0 m. L Accuracy- cannot determine without knowing the actual mass Precision- Somewhat precise; each number is semi-close to the average D. E. F. Using the average mass and volume, calculate the density of the unknown metal. D = m/V D = 175. 5 g / 65. 0 m. L D = 2. 70 g/m. L The table below shows the density of various metals. Aluminum What is the most likely identity of the unknown metal? How accurate was the average density compared to the actual density? 100% accurate
 Energy naturally flows from warmer matter to cooler matter
Energy naturally flows from warmer matter to cooler matter Unit 2 matter and energy
Unit 2 matter and energy Meysam golmohammadi
Meysam golmohammadi Label the cranial dura septa and associated sinuses.
Label the cranial dura septa and associated sinuses. Gray matter and white matter
Gray matter and white matter Telecephalon
Telecephalon Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Chemistry matter and its changes
Chemistry matter and its changes Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chapter 10 assessment the mole answer key
Chapter 10 assessment the mole answer key Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chemistry matter and change chapter 6
Chemistry matter and change chapter 6 Chemistry matter and change chapter 10
Chemistry matter and change chapter 10 Chemistry matter and change answer key chapter 2
Chemistry matter and change answer key chapter 2 Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Section 1 composition of matter
Section 1 composition of matter Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Examples of matter in chemistry
Examples of matter in chemistry Matter flowchart
Matter flowchart Matter flowchart chemistry
Matter flowchart chemistry Graphic organizer about matter
Graphic organizer about matter Non examples of homogeneous mixture
Non examples of homogeneous mixture Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Foods that are heterogeneous mixtures
Foods that are heterogeneous mixtures Ib chemistry organic chemistry
Ib chemistry organic chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Lesson 3 energy and matter in ecosystems answer key
Lesson 3 energy and matter in ecosystems answer key Section 16.1 thermal energy and matter
Section 16.1 thermal energy and matter Section 1 matter and thermal energy
Section 1 matter and thermal energy Science matter and energy
Science matter and energy Matter energy and measurement
Matter energy and measurement Dark matter and dark energy ppt
Dark matter and dark energy ppt What are trophic levels
What are trophic levels Lesson 1 thermal energy and the behavior of matter
Lesson 1 thermal energy and the behavior of matter Matter and energy concept map
Matter and energy concept map Phases of matter foldable
Phases of matter foldable Mechanical waves and electromagnetic waves similarities
Mechanical waves and electromagnetic waves similarities Kesler science sound waves answer key
Kesler science sound waves answer key A graphical model of energy flow in a community
A graphical model of energy flow in a community Unit 6 review questions
Unit 6 review questions Unit 2 matter and change
Unit 2 matter and change Ap chem spontaneity entropy and free energy
Ap chem spontaneity entropy and free energy Type of wave
Type of wave Wave transfer matter
Wave transfer matter Thermal energy in states of matter
Thermal energy in states of matter A rhythmic movement that carries energy
A rhythmic movement that carries energy Flow of energy vs flow of matter
Flow of energy vs flow of matter A repeating disturbance that transfers energy
A repeating disturbance that transfers energy What is heat energy?
What is heat energy? Thermal energy in states of matter
Thermal energy in states of matter Internal energy of matter
Internal energy of matter A loop of relatively cool incandescent gas
A loop of relatively cool incandescent gas A wave is a disturbance that transmits
A wave is a disturbance that transmits Transverse wave energy transfer
Transverse wave energy transfer Are atoms the smallest unit of matter
Are atoms the smallest unit of matter What is the basic unit of matter?
What is the basic unit of matter? Smallest unit of matter
Smallest unit of matter