Unit 1 Lecture 4 SoluteSolvent Interactions The student






- Slides: 10

Unit 1 Lecture 4: Solute/Solvent Interactions The student is able to draw and/or interpret representations of solutions that show the interactions between the solute and solvent.

Mixtures • A physical blend of two or more pure substances • in any proportion • each substance retains its individual properties • can be separated by physical means.

Two Type of Mixtures 1. Heterogeneous – species retain their individual properties and do not mix smoothly throughout – Suspensions – have large particles that if left undisturbed will settle out (muddy water, paint) – Colloids – have very small particles that do not settle out (milk, blood) 2. Homogeneous – solution where solutes completely dissolve into a solvent and individual species are indistinguishable. – Solutions - Can exist in the solid, liquid or gaseous phase depending on the state of the solvent

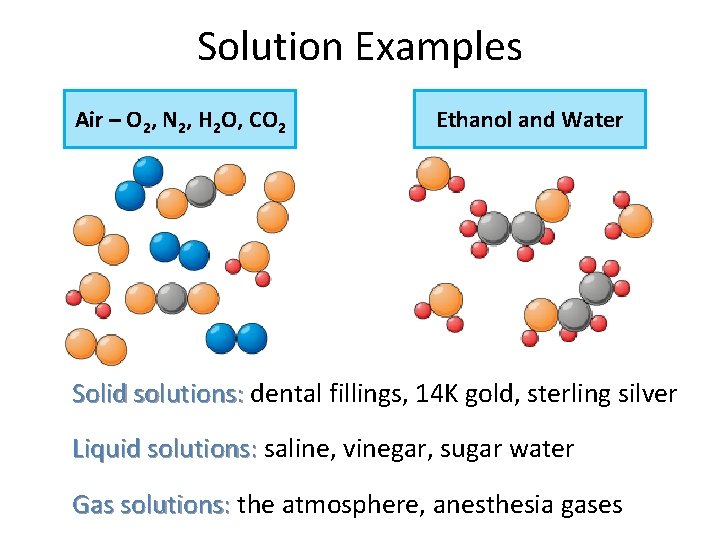
Solution Examples Air – O 2, N 2, H 2 O, CO 2 Ethanol and Water Solid solutions: dental fillings, 14 K gold, sterling silver Liquid solutions: saline, vinegar, sugar water Gas solutions: the atmosphere, anesthesia gases

Solvent • Substance that dissolves a solute to form a solution; most plentiful chemical species in the solution

Solute • One or more chemical species dissolved in a solution.

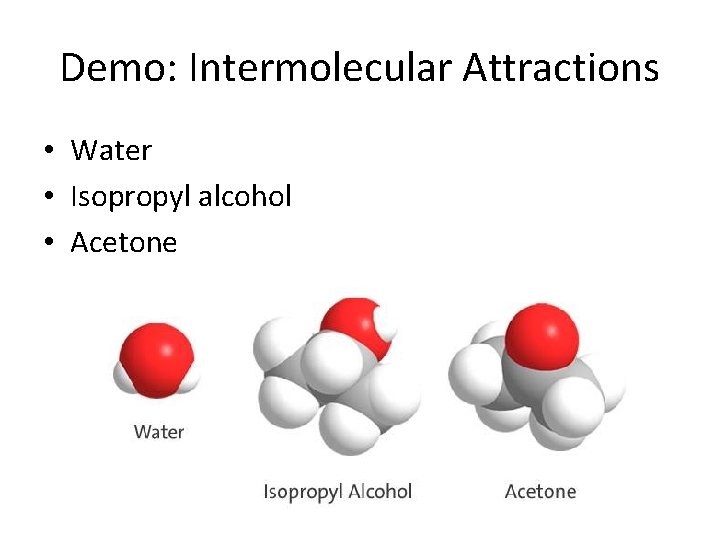
Demo: Intermolecular Attractions • Water • Isopropyl alcohol • Acetone

Mini-Labs • Get into 2 lab groups and begin performing the mini-lab experiments. • You have 20 minutes to complete the activity. • Each team will briefly summarize their findings to the class and describe their particular phenomenon.

Boiling Point Elevation • Boiling occurs when the pressure of the evaporating solution is equal to atmospheric pressure. • Solvent particles interact with the solute particles causing fewer solvent particles from escaping the solution. • More energy is needed to cause the solution to boil.

Freezing Point Depression • Freezing occurs when solvent particles arrange and organize themselves in an ordered solid. • Solute particles get in the way of solvent particles preventing the solution from freezing. • Colder temperatures are required to cause the solution to freeze.