Unit 1 Introduction to Physical Science Scientific Notation













![Metric Stairs q You should be comfortable with converting from [cm] to [m], [mm] Metric Stairs q You should be comfortable with converting from [cm] to [m], [mm]](https://slidetodoc.com/presentation_image_h/14c00088f840a23d425b38d4dcc2041b/image-19.jpg)




























- Slides: 60

Unit 1 – Introduction to Physical Science

Scientific Notation

• What is the SI unit for: – volume? – Mass? – Length? – Temperature? – Time? • What does the prefix kilo mean? • Convert 50 m to cm. • Convert 2000 mg to kg.

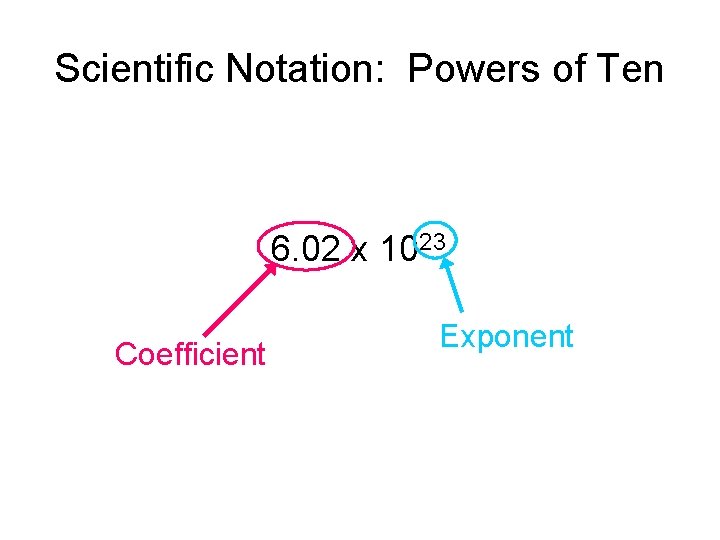
Scientific Notation: Powers of Ten 6. 02 x 1023 Coefficient Exponent

Scientific Notation: Powers of Ten Consider the number 123, 000, 000 Move the decimal point after the first digit, making the number have a value between 1 and 10. 1. 2300000 To find the exponent, count the number of places from the end of the number to where the decimal was placed.

Scientific Notation: Powers of Ten Large number (>1) = positive exponent Small number (<1) = negative exponent DO YOU UNDERSTAND THIS? !?

Scientific Notation: Powers of Ten So, for a very small number. . . 0. 000 0144 Visual display of powers of ten (a view from outer space to the inside of an atom)

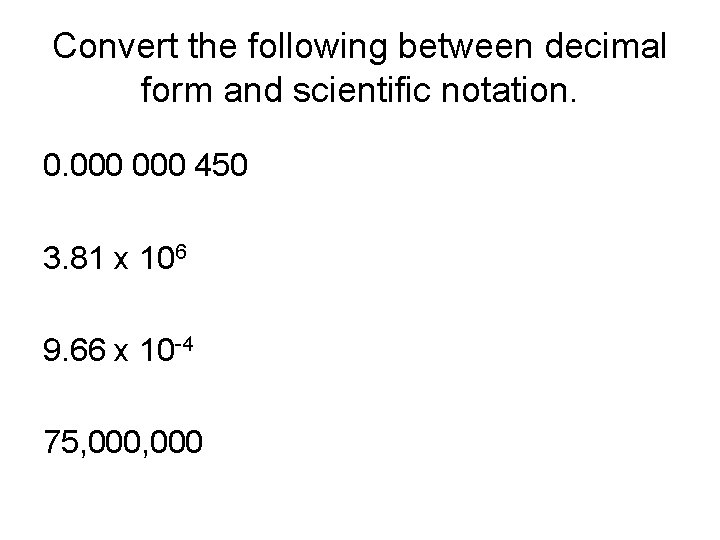
Convert the following between decimal form and scientific notation. 0. 000 450 3. 81 x 106 9. 66 x 10 -4 75, 000

Math in Physical Science Worksheet • Please do the algebra practice and Scientific Notation conversions and then we’ll look at metric units.

1. What is the measuring system that scientists worldwide use? 2. Which is larger a kilometer or a centimeter? 3. What unit is used to measure mass? 4. What unit is used to measure volume? 5. What unit is used to measure length? 6. What is the formula for measuring the volume of a solid?

A Common System for Trade English system of measurement originated in 1215 with the signing of the Magna Carta. It attempted to bring uniform measurements to world trade. In 1790, the French government appointed a committee of scientists to develop a universal measuring system. It took ~10 years, and they unveiled the Metric system, now known as the International System of Units.

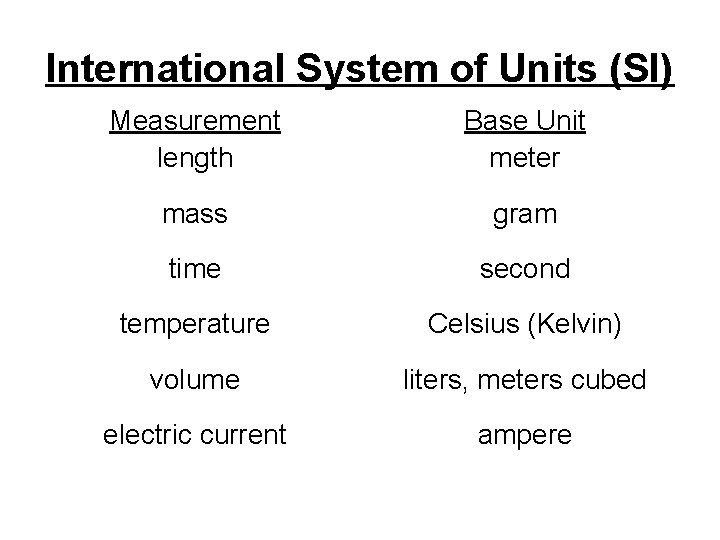
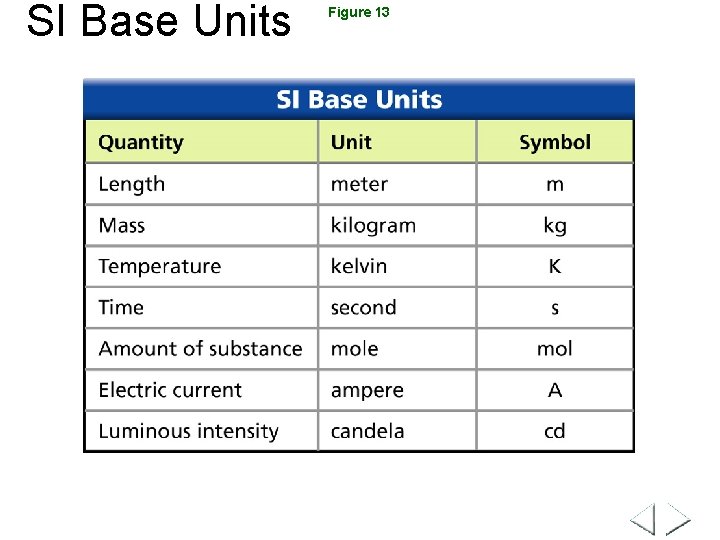
International System of Units (SI) Measurement length Base Unit meter mass gram time second temperature Celsius (Kelvin) volume liters, meters cubed electric current ampere

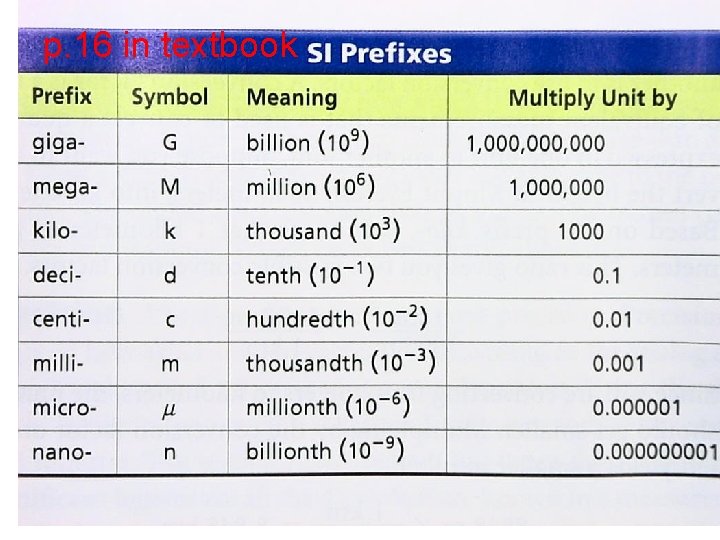
International System of Units (SI) Also includes prefixes that are placed on base units All prefixes represent a power of ten, or movement of the decimal one position. k h D U d c m

No Cussing! The following 4 -Letter words are forbidden here: Inch Foot Yard Mile Pint Acre And we NEVER use the F word (useo. C) Please keep it clean and Metric

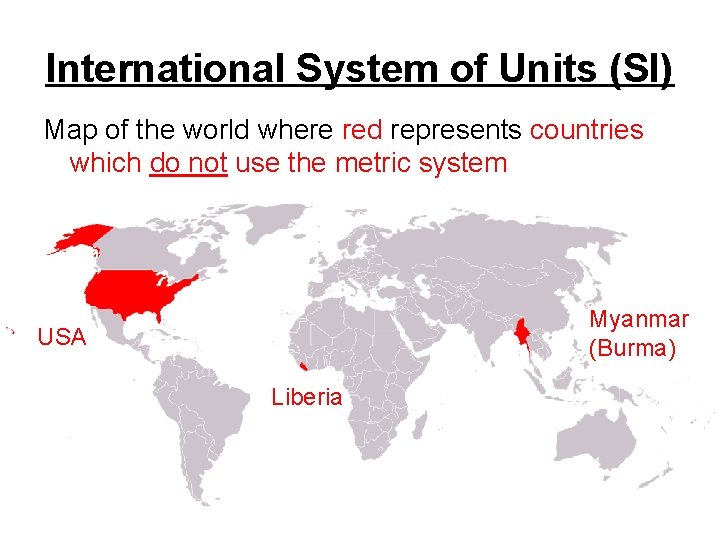
International System of Units (SI) Map of the world where red represents countries which do not use the metric system Myanmar (Burma) USA Liberia

SI Base Units Figure 13

International System of Units • “SI” – Based on the number 10 – Distance (length) uses meter (about 39 inches) – Mass (how much matter) uses gram ( a nickel is about 5 grams) – Volume (how much space) • Liquid volume – uses liter ( a little more than a quart) • Solid volume – uses cm 3 ( about the size of a sugar cube) • 1 ml = 1 cm 3 – Weight (affect gravity has on object) uses newton ( an apple weighs about 1 newton) (1 pound is about 4. 4 newtons) – Density = Mass / Volume = g/L

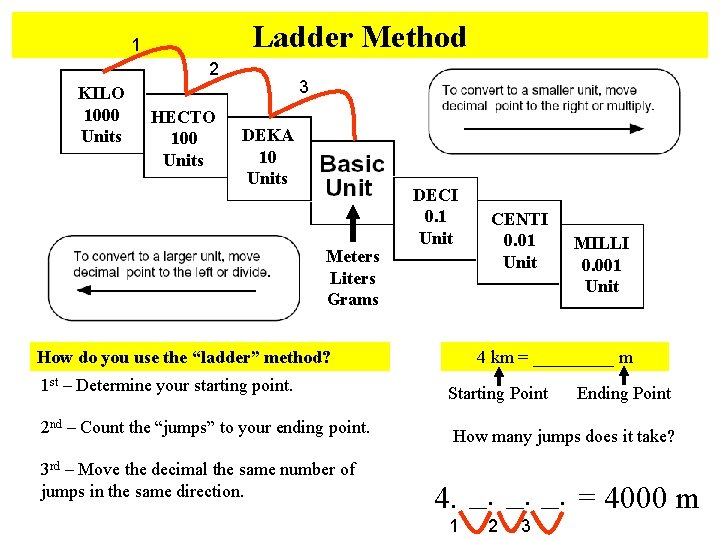
To Amplify the Point • Distances can be short or very long. – Basic metric unit of length is the meter. – Metric prefixes are based on the number 10. – 10 meters = 1 dekameter – 10 dekameters = 1 hectometer – 10 hectometer = 1 kilometer – Therefore : 1 kilometer =1000 meters – And… – There are 10 decimeters in a meter – There are 10 centimeters in a decimeter – There are 10 millimeter in a centimeter – Therefore: 1000 millimeters = 1 meter
![Metric Stairs q You should be comfortable with converting from cm to m mm Metric Stairs q You should be comfortable with converting from [cm] to [m], [mm]](https://slidetodoc.com/presentation_image_h/14c00088f840a23d425b38d4dcc2041b/image-19.jpg)
Metric Stairs q You should be comfortable with converting from [cm] to [m], [mm] to [km], and so on. Convert: 1527 centigrams into hectograms: going four steps up means you move the decimal 4 places to the left. Therefore: 1527 centigrams =. 1527 hectograms & 9. 8712345 kg = (steps to the right) 9871234. 5 mg

So remember: • • Kilo = 1000 Hecta = 100 Deka = 10 Base = 1 Centi = 1/10 Deci = 1/100 Milli = 1/1000

Remember this Saying: King Henry Died By Drinking Chocolate Milk

• • • Kilometer Hectometer Decameter Meter Liter Gram decimeter centimeter 1 pound = 453. 59 grams millimeter decimilli centimilli Micrometer Nanometers are 3 more spaces over!!

• How many micrometers are in 10 millimeters? • Remember to always start with what you have and go to what you need to find out! • K h d m d c m • If you move to the right on the chart, you will move your decimal to the right.

Ladder Method 1 2 KILO 1000 Units HECTO 100 Units 3 DEKA 10 Units Meters Liters Grams DECI 0. 1 Unit How do you use the “ladder” method? 1 st – Determine your starting point. 2 nd – Count the “jumps” to your ending point. 3 rd – Move the decimal the same number of jumps in the same direction. CENTI 0. 01 Unit MILLI 0. 001 Unit 4 km = _____ m Starting Point Ending Point How many jumps does it take? 4. __. __. = 4000 m 1 2 3

• How many millimeters are in 10 meters? • 10, 000

• How many meters are in 1 kilometer? • 1, 000

• How many millimeters are in 1 micrometer? • 1000

p. 16 in textbook

Tools and Measurement Lab • Complete the activity on your own paper – 1 paper for the group • When finished: – return everything to the basket or middle of the table – turn in answer sheet and worksheet – return to your seats


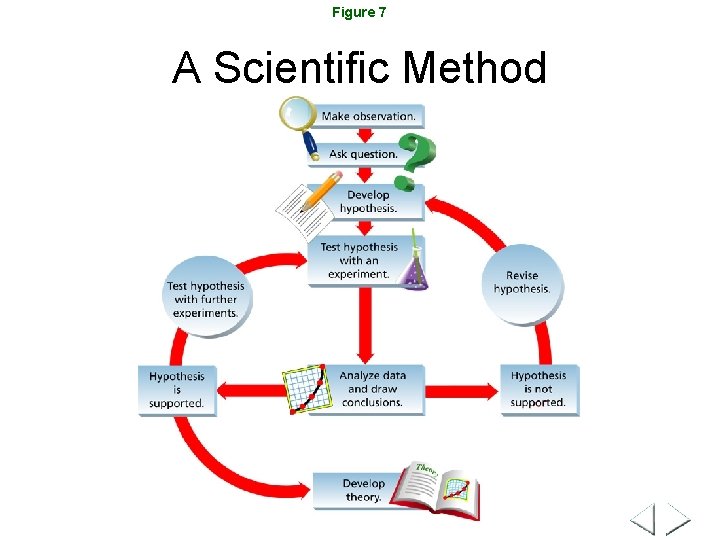
Figure 7 A Scientific Method

Steps of the Scientific Method • • Purpose Research Hypothesis Experiment Analyze data Form conclusion Repeat

Experiment vocabulary Variable – things that change Constants things that stay the same

Variables • The factor that is changed purposefully to be tested is known as the independent variable. (Ask yourself, what am I changing? ) • The factor that is measured or observed is called the dependent variable.

What is the Purpose of a Control? • Control groups are NOT being tested (it’s the one you don’t do anything to) • Controls are used for COMPARISON • Experimental group - variables are changed

Theory vs. Law • Theory: when many experiments show the same conclusion, the hypothesis can become a theory. • Theories are NOT proven!! • Law: fact of nature ex. gravity

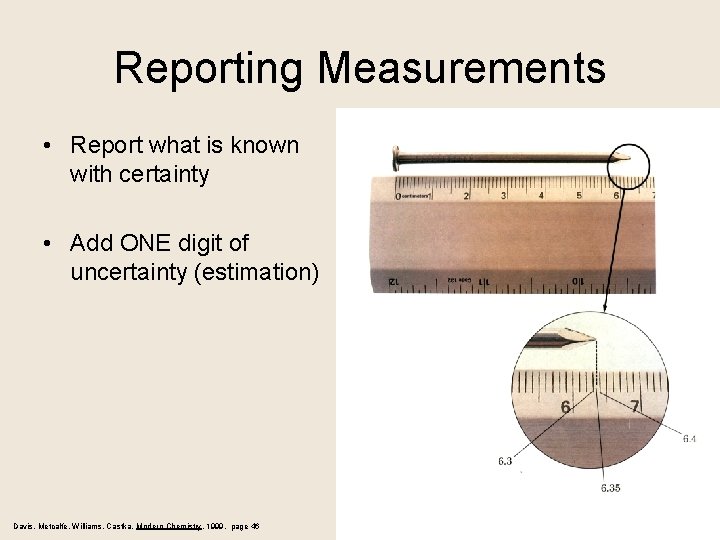
Reporting Measurements

Reporting Measurements • Report what is known with certainty • Add ONE digit of uncertainty (estimation) Davis, Metcalfe, Williams, Castka, Modern Chemistry, 1999, page 46

What would you report as the length of each pin shown below? Zumdahl, De. Coste, World of Chemistry 2002, page 122

What is the temperature as measured by each thermometer shown below? 4. 0 o. C 10 10 100 5 5 50 0 8. 3 o. C 64 o. C 5 0 3. 5 o. C

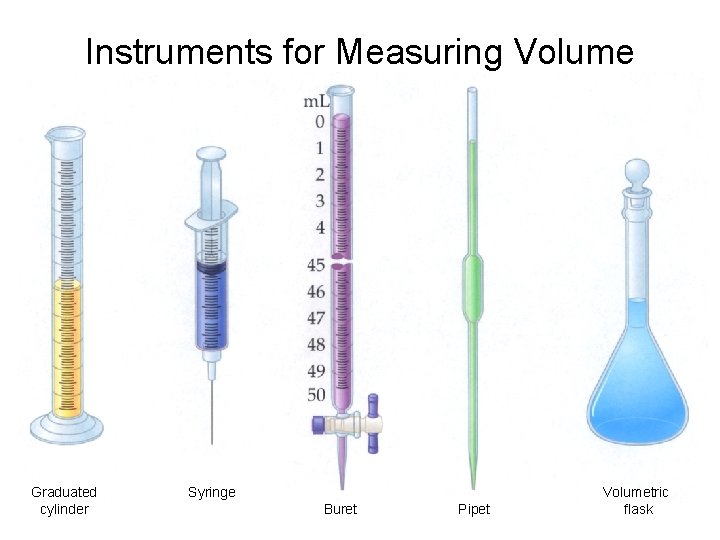
Instruments for Measuring Volume Graduated cylinder Syringe Buret Pipet Volumetric flask

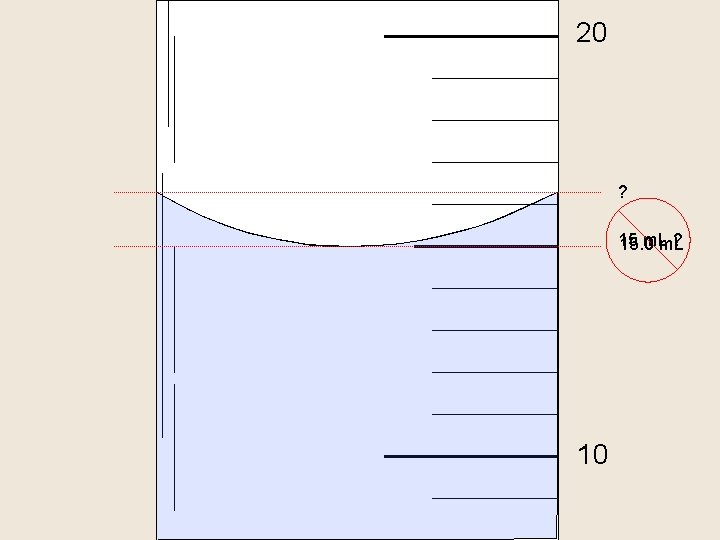
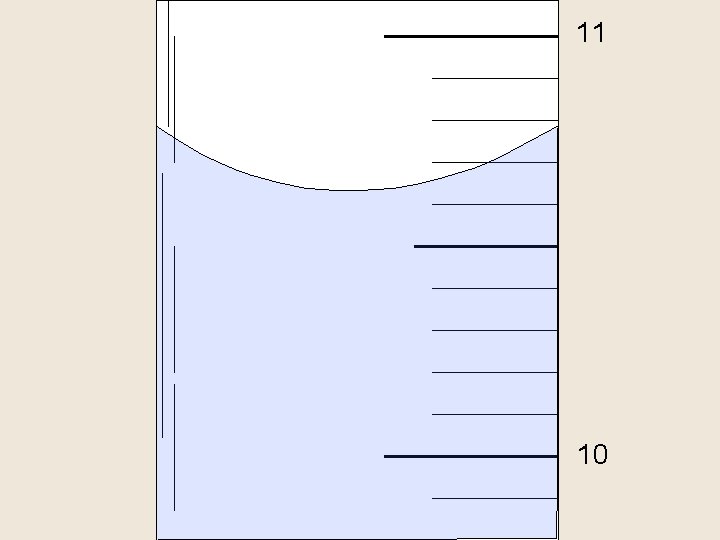
Reading a Meniscus 10 m. L 10 line of si ght too proper line of sight high 8 f s o line graduated cylinder in reading correct ow too l ight high o o g t 6 read ing t oo lo w

20 ? 15 m. L ? 15. 0 m. L 10

20 10

11 10

• Express these numbers in Scientific Notation: . 0000045 6, 000, 000

Temperature

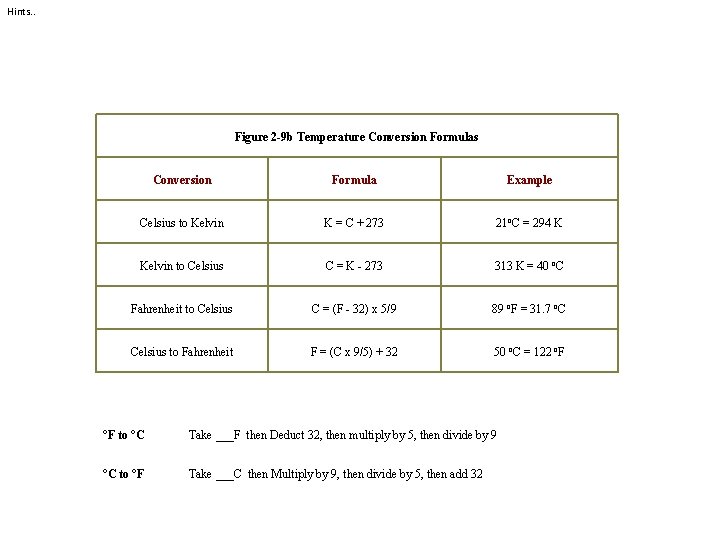
Hints. . Figure 2 -9 b Temperature Conversion Formulas Conversion Formula Example Celsius to Kelvin K = C + 273 21 o. C = 294 K Kelvin to Celsius C = K - 273 313 K = 40 o. C Fahrenheit to Celsius C = (F - 32) x 5/9 89 o. F = 31. 7 o. C Celsius to Fahrenheit F = (C x 9/5) + 32 50 o. C = 122 o. F °F to °C Take ___F then Deduct 32, then multiply by 5, then divide by 9 °C to °F Take ___C then Multiply by 9, then divide by 5, then add 32

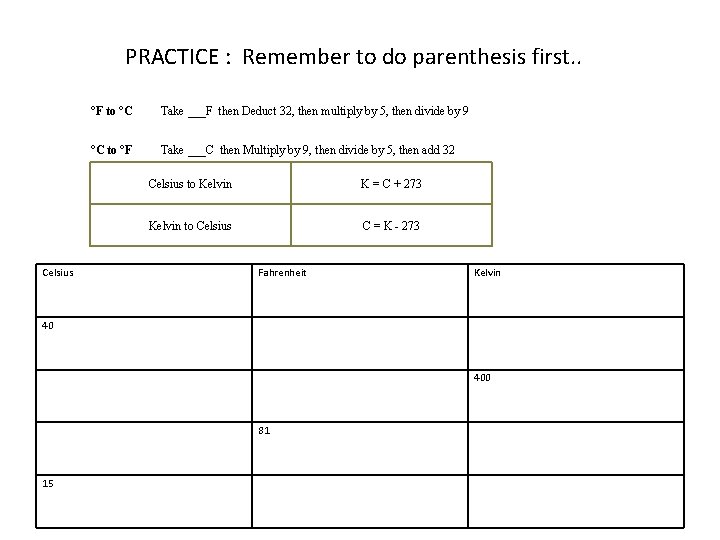
PRACTICE : Remember to do parenthesis first. . Celsius °F to °C Take ___F then Deduct 32, then multiply by 5, then divide by 9 °C to °F Take ___C then Multiply by 9, then divide by 5, then add 32 Celsius to Kelvin K = C + 273 Kelvin to Celsius C = K - 273 Fahrenheit Kelvin 40 400 81 15

Temperature • The SI temperature scale – the Kelvin Scale – is also known as the absolute scale. • 0 Kelvin is absolute zero. This is the temperature at which all molecular motion stops. There is nothing below no molecular motion. There is nothing below absolute zero Kelvin.

Temperature • On the Fahrenheit and Celsius scales, the unit is called a degree. On the Kelvin scale, the unit is a Kelvin. • Do not use a degree symbol in the Kelvin scale!

Temperature You must know how to convert between Kelvin and Celsius temperatures: K = °C + 273 So absolute zero is what Celsius temperature? -273 °C

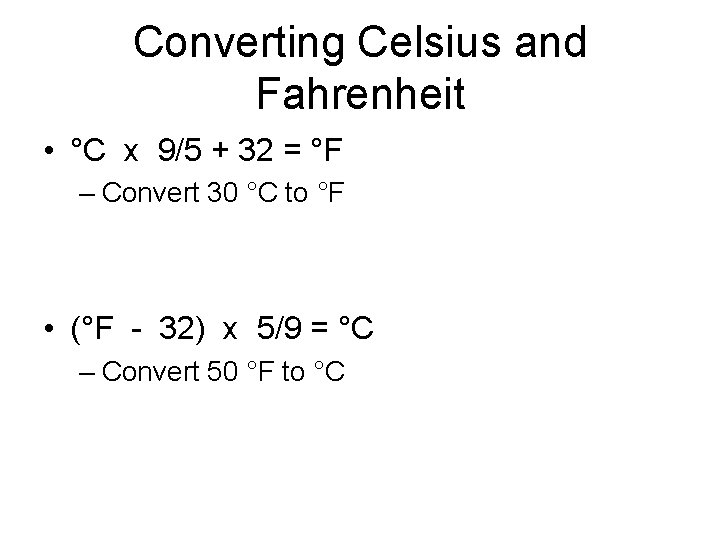
Converting Celsius and Fahrenheit • °C x 9/5 + 32 = °F – Convert 30 °C to °F • (°F - 32) x 5/9 = °C – Convert 50 °F to °C

1. What is the freezing point of water in Celsius? 2. What is the boiling point of water in Celsius? 3. Convert 100 C to K.

Density • Begins here!!

Density • measure of how tightly packed the particles of an object are • Ratio of mass to volume

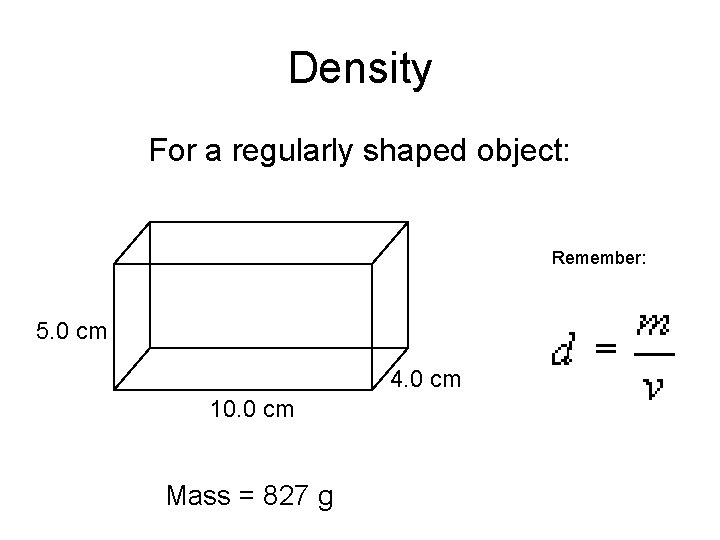
Density For a regularly shaped object: Remember: 5. 0 cm 4. 0 cm 10. 0 cm Mass = 827 g

Billiard ball in mercury

Density A rock of shiny metal has a mass of 125 g and a volume of 14. 0 m. L. What is the density of this metal?

Today’s Agenda • Density Lab • Review for Test – Scientific Method – Graphing – Scientific Notation – Metric Measurement – Density