UNIT 1 Foundations Chapter 2 MATTER Chapter 2








- Slides: 34

UNIT 1 Foundations Chapter 2 - MATTER

Chapter 2 A The Particle Model of Matter

Describing Matter • What is matter? – All substances that occupies a volume of space and has mass – Can be sensed and measured – Measurable mass, measurable weight – Made up of particles – Solid, liquid, gas, and plasma • What is plasma? – Hot, ionized gases (more to come)

The Particle Model • Four hundred years before Christ… – Greek philosophers started discussing the nature of matter – Was in continuous, was it not continuous – Called “atomos”, meaning indivisible • Particle Theory (Model) – All matter is made of exceedingly small particles • Diameter of a typical particle is measured in nanometers, very small metric unit

The Particle Model • These particles are referred to as atoms – Building blocks of matter – Combine to form molecules – The basic particle of matter from which all other matter is constructed, consisting of protons, electrons and neutrons. • How do we know? – Scientists can see them using a scanning tunneling electron microscope (STM) – Properties associated with matter • Color, density, electrical conductivity can’t be seen in each individual particle, it is how they function together

Evidence for the Particle Model • Atoms are held together by bonds – a connection by sharing or transferring electrons to form larger structures like molecules • How do we know these particles exist? – Particles can be observed by color and taste – Particles are in constant motion • Diffusion – the process of spreading out and mixing due to particle motion • Brownian motion – microscopic, random jostling of suspended matter due to the collisions of innumerable gas or liquid particles in which the matter is suspended. • All of this makes up the kinetic-molecular theory of matter.

Particle Model • Summary of the four main ideas of the particle model 1. 2. 3. 4. All matter is made of particles Particles are always moving There are spaces between particles Particles are attracted to one another • One of the most useful theories ever proposed in science • Predicts the behavior of the states of matter as well as explains pressure, diffusion and conductivity

The Atom • Let’s review what an atom is: – The basic particle of matter from which all other matter is constructed, consisting of protons, electrons, and neutrons. (subatomic particles) – Nucleus at the center – Nucleus contains protons and neutrons and makes up the mass of the atom • Proton is positively charged, neutron has no charge but the same mass as the proton • The number of protons determines the type of atom or element • Electrons are negatively charged, occupies the spherical volume and has insignificant mass compared to the proton and neutron • Electrons determine the size of the atom and are equal in number to the proton so the atom has no charge

Molecules • Molecules – Distinct particles formed when two or more atoms bond together – Can be simple like oxygen – Can be large and complex like DNA

Ions • Atoms can gain or lose electrons forming ions – An atom or molecule that has gained or lost electrons, thus producing an imbalance between the number of protons and electrons in the particle – Anions • Gained electrons, have a negative charge – Cations • Lost electrons, have a positive charge • Ions form matter and can be attracted and repelled by an electric current

Chapter 2 B Classification of Matter

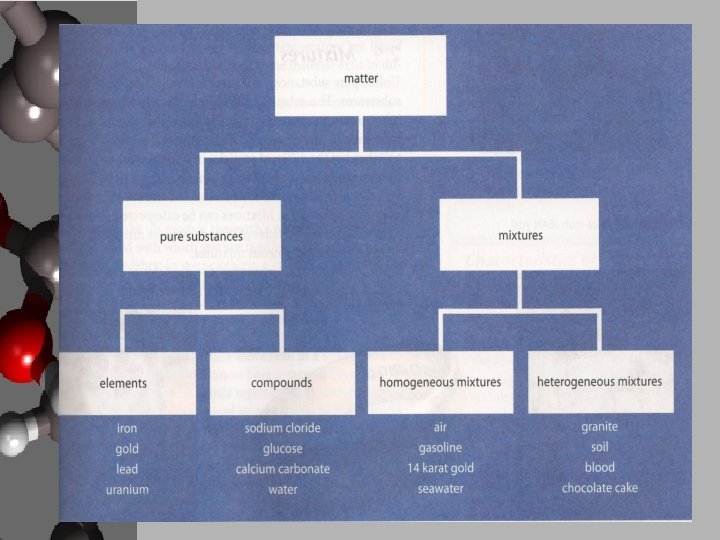
The Classification Model • Classifications help us to understand our surroundings, they organize into familiar patterns • Classification has been around since the beginning of creation – Adam named all the animals • Matter needs to be classified more than just solid, liquid, and gas

Pure Substances • Pure substances – Matter that contains only one kind of atom or a fixed ratio of different atoms 1. Element – One kind of atom containing all the same number of protons in their nuclei – Periodic table contains all known elements thus far 2. Compound – Two or more elements bonded in a fixed ratio – Inorganic – usually do not contain carbon – Organic – contain carbon and are produced by living things • Not all compounds that contain carbon are organic • Other elements present are hydrogen, nitrogen, phosphorus, oxygen

Mixtures • Mixtures – Combinations of two or more substance – Elements, compounds or other mixtures – Mixtures are not bonded together • Two types of mixtures 1. Heterogeneous • Non-uniform mixture that contains two or more distinct phases, usually of different kinds of matter 2. Homogeneous • A uniform mixture of particles of different substances that form a single phase, also called a solution


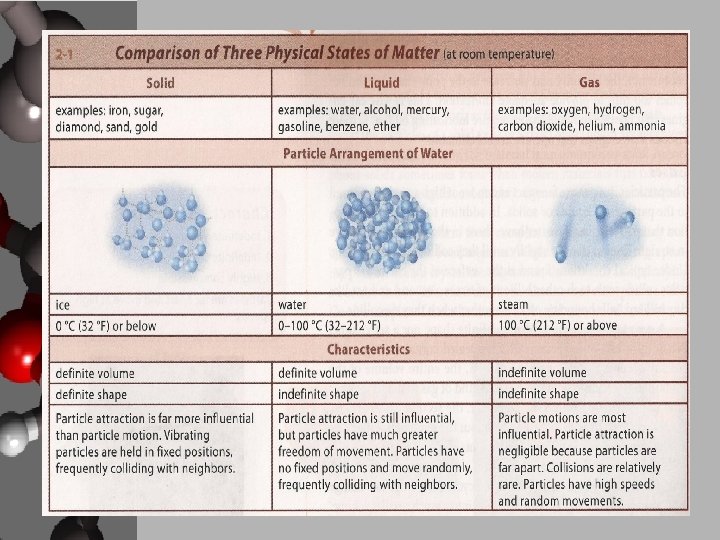
States of Matter • State • A physical form of matter determined by the arrangement and energy of its particles: solid, liquid, gas

Solids • Characteristics of a Solid – – Definite volume Definite shape, rigid Low compressibility Particles are close together and vibrate in fixed positions • Types of solids 1. Crystalline • Particles in a fixed, repeating structure • Quartz, sugar, salt 2. Amorphous • Particles arrangement is fixed, but random • Glass, wax 3. Heterogenous • Particles are fixed, some order • Wood, bone

Liquids • Characteristics of a Liquid – Definite volume – No definite shape, assume the shape of the container they are in – Forms a free surface if it does not completely fill its container – Low compressibility – Particles are completely mobile but still close together due to mutual attraction – Collide randomly and vibrate individually – Exhibit properties of viscosity • A measure of a fluid’s resistance to flow, the “thickness” of a liquid – lubricants

Gases • Characteristics of a Gas – Indefinite volume – Indefinite shape – Highly compressible – Particles are far apart and move at high speeds – Collide in straight lines and vibrate individually – Gases can flow! – Fluids • A substance that can flow, a liquid or gas – Gas pressure • The pressure a gas exerts on its container or on an object immersed in it


Chapter 2 C Changes in Matter

Physical Properties and Changes • Physical property – Any feature that can be observed or measure without altering the kind of matter being studied – Examples are color, density, hardness, crystalline form, electrical conductivity, texture, physical state – Tells us what type of matter is better to use • A diamond drill bit, or a quartz drill bit? • Physical change – A change that does not alter the composition of the material – Particles can rearrange, but not change – Water, wood, sugar

Chemical Properties and Change • Chemical properties – A feature of a substance that describes how its chemical identity changes in the presence of another substance • Iron • Stainless steel • Chemical Change – Any change in a substance that alters its composition, the kind and ratio of elements in the substance – Also referred to as a chemical reaction – Bonds are broken and new ones formed

Using Properties of Matter to Solve Problems • Bulletproof Vests – Made of Kevlar because of its physical property of strength – Five times that of steel – The strength comes from the chemical properties of its molecular structure – Made of long polymers consisting of smaller repeating groups of atoms – Loses strength in the presence of water or ultraviolet light so it is covered with a protective substance

Conservation of Matter • Law of Conservation of Matter – Matter can be neither created nor destroyed, but only changed from one form to another – Antoine Lavoisier is credited with being the first to confirm this law in 1785

Nuclear Changes • In physical and chemical changes the kinds of atoms involved always remain the same • Nuclear change – The change in the energy or composition of an atom’s nucleus when it emits or absorbs nuclear radiation – Forms totally different atoms – Takes place in nuclear power plants and nuclear bombs

Chapter 2 D Changes of State

Changes of State and Temperature • Phase change or change of state – When matter changes from one state to another, especially when two or more states are present at the same time • Motion of particles is directly related to the temperature – One goes up so does the other • Thermal energy – Related to the random vibrations and motions of the particles in a substance

Melting and Freezing • Melting – The change of state from a solid to a liquid when thermal energy is absorbed • Melting point – The temperature at which a pure solid turns into a liquid at 1 atm; the same temperature as the freezing point • Freezing (solidifying) – The change of state from a liquid to a solid when thermal energy is released • Freezing point – The temperature at which freezing occurs at 1 atm; the same temperature as the melting point

Vaporization and Condensation • Vaporization – Any process in which particles of a liquid enter the gaseous phase • Vapor – The gaseous phase of a substance – What’s the difference between a gas and a vapor? • Vapor pressure • The gas pressure exerted on the surface of a liquid by its vapor in a close container when the gas and liquid are in equilibrium

Vaporization and Condensation • Three types of vaporization 1. Boiling • Rapid vaporization that occurs when a liquid’s vapor pressure equals or exceeds atmospheric pressure and the static pressure in the liquid, forming vapor bubbles • Boiling occurs at the boiling point and throughout the whole liquid • The temperature and pressure at which boiling occurs

Vaporization and Condensation 2. Evaporation Relatively slow vaporization that occurs when a liquid’s temperature is below its boiling point, but above its freezing point Evaporation occurs at the liquid’s surface A method of cooling • • Sweating

Vaporization and Condensation 3. Sublimation – The change of state from a solid directly to a vapor at temperatures below the melting point of the substance – Dry ice, mothballs, solid air fresheners – Deposition(not in book) • The change of state from a gas(vapor) directly to a solid

Vaporization and Condensation • Condensation – The change of state from gas to liquid when thermal energy is released to the surroundings – Opposite of vaporization – Dew