Unit 1 Classifying Matter Notes Flow chart Pure
















- Slides: 25

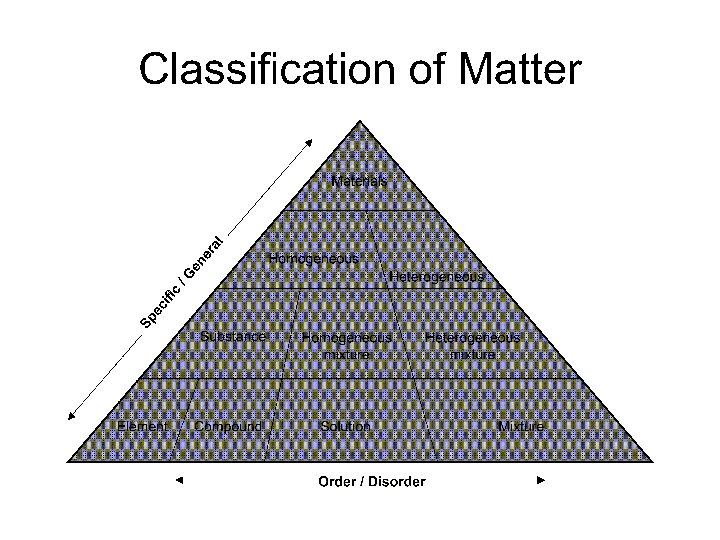
Unit 1 Classifying Matter Notes

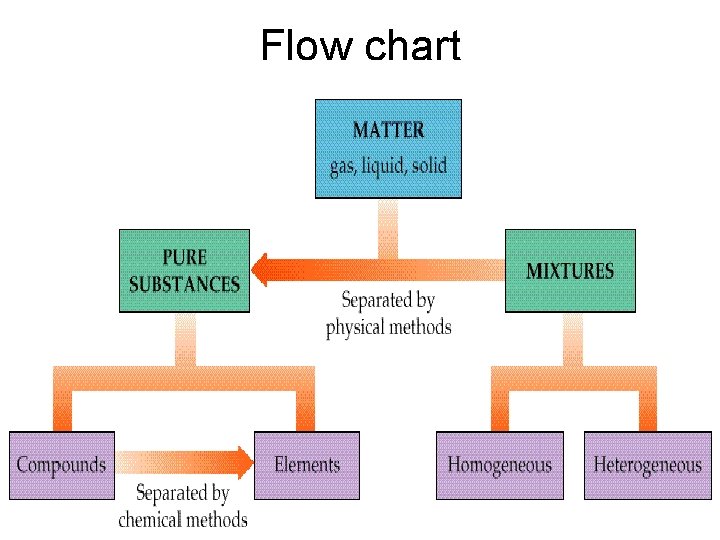
Flow chart

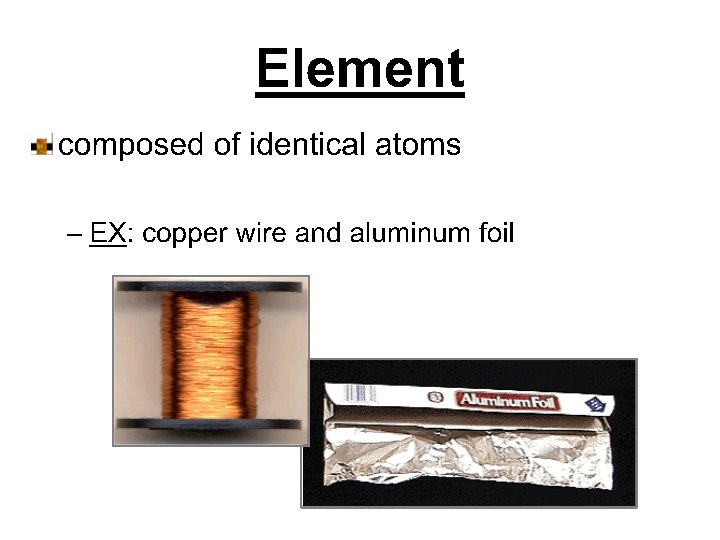
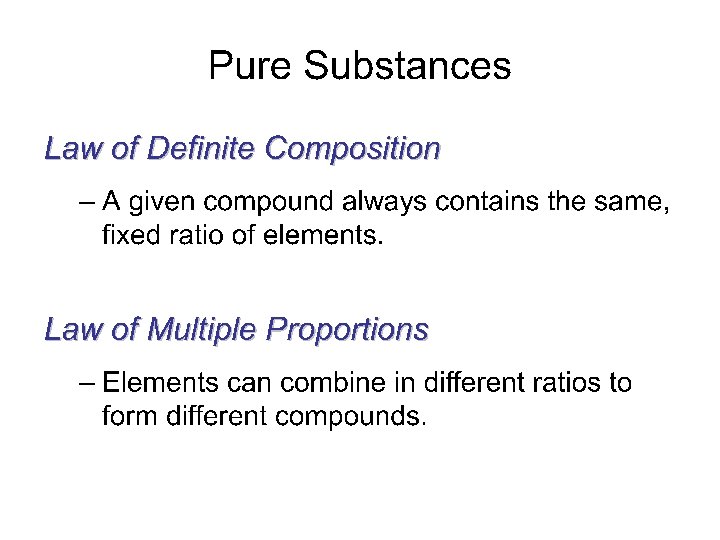
Pure Substances (elements and compounds) 1. EVERY SAMPLE of a given pure substance has EXACTLY THE SAME characteristic properties. a. Boiling point b. Freezing point c. Melting point d. Density 2. Can NOT BE SEPARATED by PHYSICAL means Ex: water always has a boiling point of 100 C, melting point of 0 C, freezing point of 0 C and a density of l. 00 g/ml. Other pure substances have their own individual characteristics (bp, mp, fp, density)




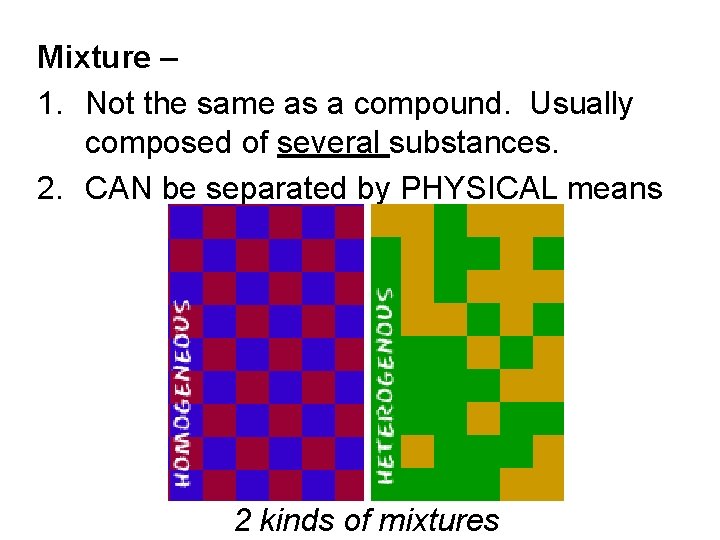
Mixture – 1. Not the same as a compound. Usually composed of several substances. 2. CAN be separated by PHYSICAL means 2 kinds of mixtures


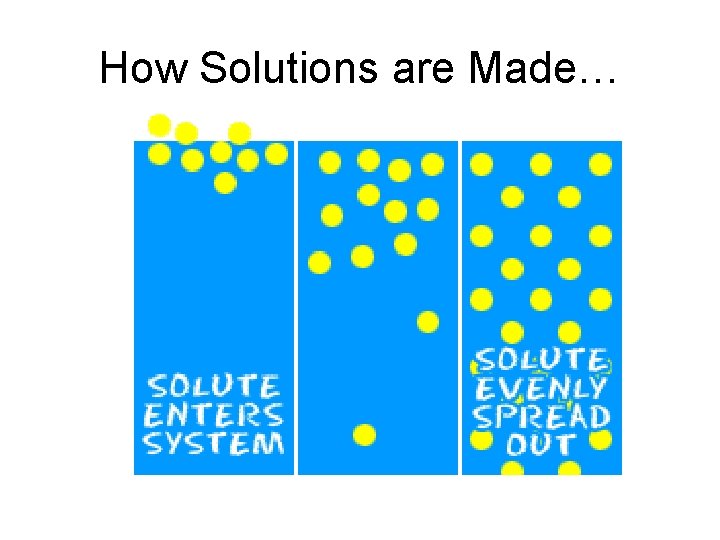
1. Homogeneous mixtures – also called solutions have the same composition throughout. Ex: salt water


How Solutions are Made…



2. Heterogeneous mixtures – the composition is NOT the same throughout; Ex: blue cheese dressing, sand water, trail mix


Learning Check: Identify the following as pure substances (p) or mixtures (m): P Oxygen (O 2) ___ M Lemonade ___ M Snickers bar ___ P Distilled water ___ P Dry ice ___ M Soil ___

Learning Check: Classify the following as Homogeneous (ho) or Heterogenous mixtures (he) Ho Pure Air ___ He Salsa ___ He Chocolate chunk ice cream ___ Ho Ink ___ Ho Blood ___ He Chicken noodle soup ___

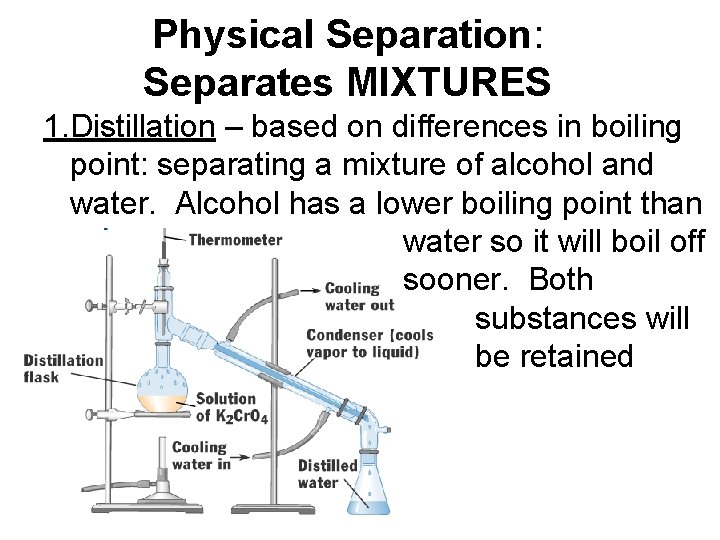
Physical Separation: Separates MIXTURES 1. Distillation – based on differences in boiling point: separating a mixture of alcohol and water. Alcohol has a lower boiling point than water so it will boil off sooner. Both substances will be retained

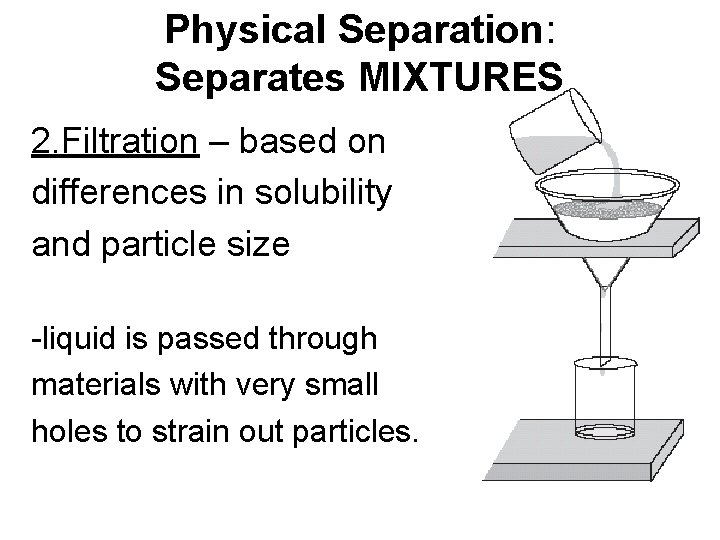
Physical Separation: Separates MIXTURES 2. Filtration – based on differences in solubility and particle size -liquid is passed through materials with very small holes to strain out particles.

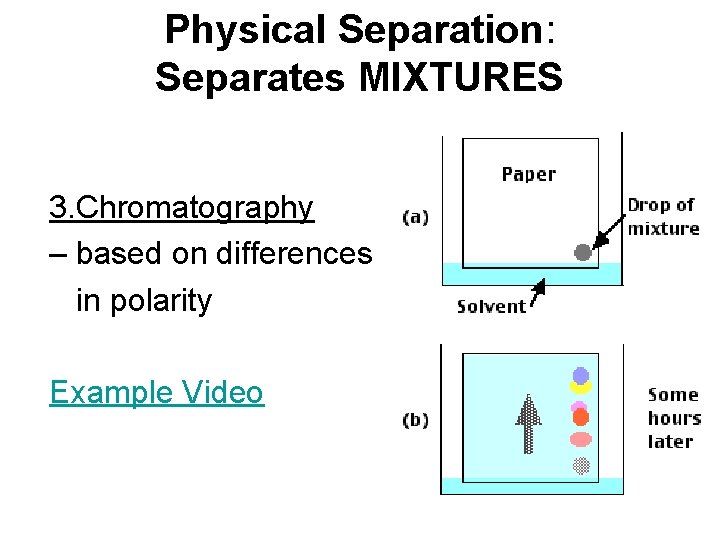
Physical Separation: Separates MIXTURES 3. Chromatography – based on differences in polarity Example Video

Physical Separation: Separates MIXTURES 4. Magnetism – separating iron and sand

Chemical Separation: Separates COMPOUNDS Electrolysis – a method of separating compounds (pure substances). This is NOT a physical separation, but a chemical separation using electricity. Water hydrogen + oxygen

Learning Check Which method would be appropriate for separating these mixtures? Filtration ________ Sawdust and water ________ Nails and dirt Magnetism ________ Water and vinegar Distillation

