Unit 1 Chemistry of Life Topic 1 Nature




























- Slides: 29

Unit 1: Chemistry of Life Topic 1: Nature of Matter Topic 2: Bonding and Properties of Water

Topic 1 The Nature of Matter

What are your cells made of? Levels of Organization in Biology Biochemistry Anatomy Ecology

OBJ 1, 2 What are your cells made of? • 80% Water! • 20% Organic compounds – Carbon – Hydrogen – Oxygen – Nitrogen – Phosphorus – Sulfur SPONCH • Trace Elements – Iron, iodine, fluoride…. .

OBJ 3, 4 What makes atoms of different elements different? • Differing numbers of Protons, Electrons and Neutrons • Atomic number and mass number (atoms) (Molecules)

Ions and Isotopes • Ion - charged atom – Cation - positive, electrons lost – Anion - negative, electrons gained • Isotope - atoms of the same element but different mass – Neutron numbers different – Ex. Carbon-12 and Carbon 14 Obj 5

Bohr model of the Atom and Structural Formulas Bohr model of Atom Structural Formulas of Molecules Alanine (Compound) Chlorine (Element) Molecules are 3 D • Specific shape • Remember VSEPR Glucose (Compound)

Topic 2 Bonding and Properties of Water

OBJ 6 Bonding • Ionic Bonds • Electron transfer to fill valance shell • Ions - charged atoms • Covalent Bonding • Electrons are shared to fill valance shells • Sharing is not always equal - Electronegativitiy

OBJ 6 Polar Molecules • Molecule acts like a magnet with positive and negative poles • Polar molecules form hydrogen bonds with each other, giving special properties • Water is involved in as many as 4 Hbonds

OBJ 7 Properties of Water • Polarity (within each molecule of water) – Uneven distribution of electrons – Greater chance of finding electrons at one end of the molecule – Due to large differences in electronegativity between the atoms – ENOxygen = 3. 5 – ENHydrogen = 2. 1 – Difference = 1. 5 (strong polar covalent)

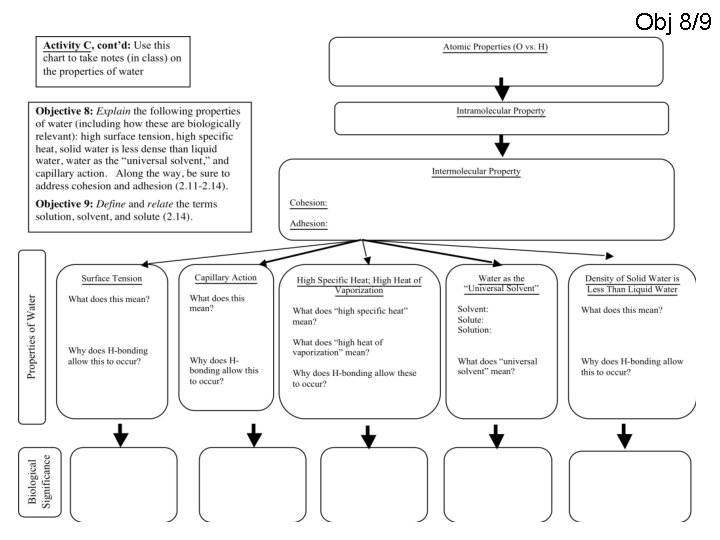
Obj 8/9

OBJ 8 Cohesion • Attraction between molecules of the same substance, often water • Cause molecules on surface to be drawn inward, forming a bead

OBJ 8 Cohesion • Surface tension allows insects and spiders walk on surface • Spread their weight over a large area

OBJ 8 Basilisk lizard! • Surface tension and rapid movement

OBJ 8 Adhesion • Attraction between molecules of different substances • Capillary action - causes water to rise in a narrow tube against force of gravity Water conducting cells Figure 3. 3 100 µm

OBJ 8 Water has a High Specific Heat • 4. 186 J/g. C • Absorbs a lot of heat • Keeps coastal climates moderate (heat sink)

OBJ 8 Water has a high heat of vaporization • Sweat cools your body as it turns to vapor

OBJ 8 Ice! • Ice is less dense than liquid water • It floats! Density = Mass/Volume

OBJ 8 Why does ice float? Hydrogen bond Ice Liquid water Hydrogen bonds are stable Hydrogen bonds constantly break and re-form

“Universal solvent” • Substance that can dissolve many different solutes. – Water - highly polar which means it can dissolve many things solute solvent solution Obj 9

Obj 9

Obj 8/9

Topic 3 Basic Chemistry

Acids and Bases • Acid – produces H+ in solution – Ex HCl • Base – produces OH- in solution – Ex Na. OH OBJ 10

p. H scale • Measure of the concentration of H+ compared to OH • Human blood = ? ? OBJ 10

p. H in Biology • Ecology– Acid rain • Biochemistry – acid and bases can be highly reactive with organic compounds • Anatomy – Blood is a buffer – Stomach p. H

Activity D – Mini p. H Lab • Groups of 2 • 3 separate experiments – water, buffer, milk – 10 m. L of substance into beaker – Add drops Na. OH – Check p. H with test strips – Add your data to class data table – Record class averages – Sketch graph using class data • Clean up – Waste beaker on front table – Rinse equipment – Throw away trash

Obj 11 Chemical Reactions in Biology C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O Products Reactants Reversible reactions – Equilibrium H+ + HCO 3 - H 2 CO 3 H 2 O + CO 2 Dehydration synthesis and Degradation Hydrolysis