Unit 1 Chemistry of Life Ch 2 Chemical




















- Slides: 44

Unit 1 Chemistry of Life Ch. 2 Chemical Context of Life Review Material is in BLUE

Instructional Links used in Unit 1 • You. Tube Channels used in Lesson 1 o Bozeman Science History of the Atoms & Periodic Table “Elements & Molecules” “Tour the Periodic Table”

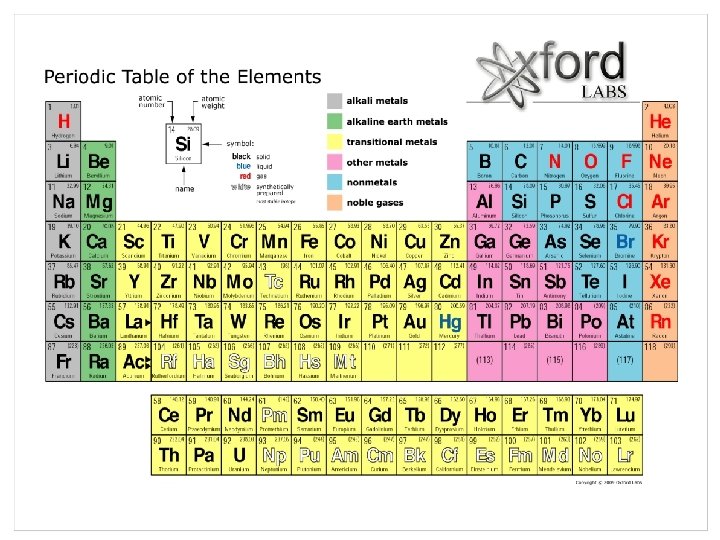
Concept 2. 1 Matter Consists of Chemical Elements in Pure Form and in Combinations Called Compounds • Matter = anything that has mass & takes up space (aka. has volume) • Elements and Compounds Elements can’t be broken down to other substances by chemical reactions 92 Elements in nature Compound = consists of 2 or more different elements in a fixed ratio; they have emergent properties (different than the elements that make them up)

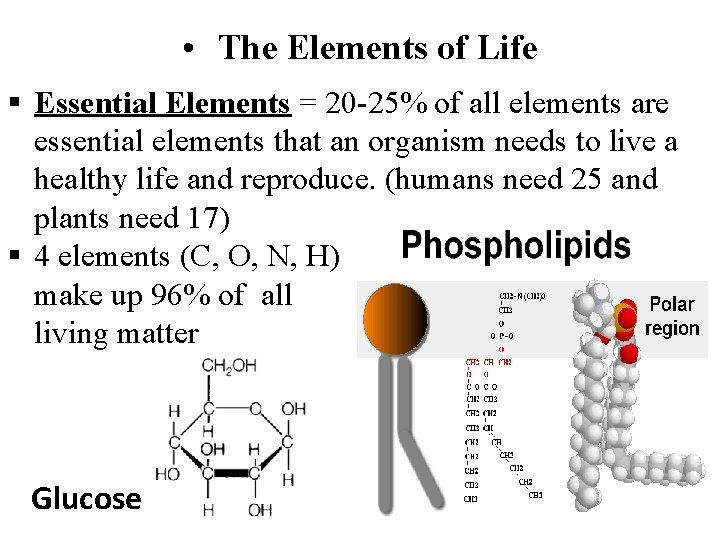
• The Elements of Life Essential Elements = 20 -25% of all elements are essential elements that an organism needs to live a healthy life and reproduce. (humans need 25 and plants need 17) 4 elements (C, O, N, H) make up 96% of all living matter Glucose

Continued… Ca, P, K, S and a few others make up the other 4%. Trace elements = required by organisms in minute amounts Table 2. 1 on page 32 shows all elements in the human body Some natural occurring elements are toxic to organisms (ie. arsenic)

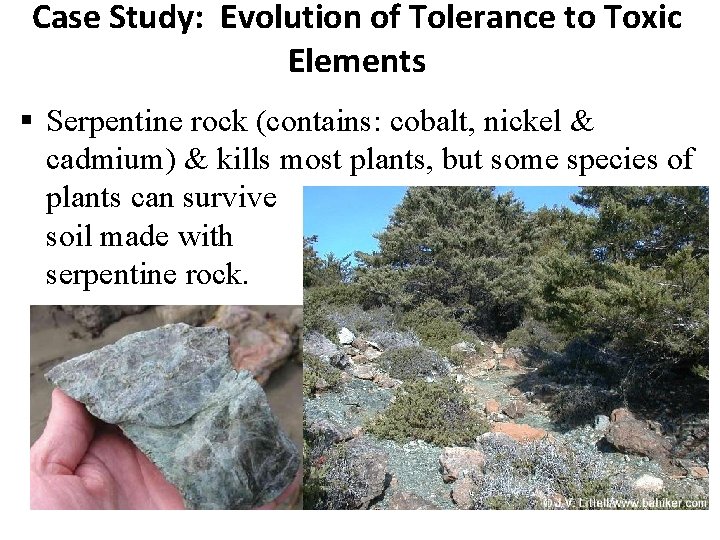
Case Study: Evolution of Tolerance to Toxic Elements Serpentine rock (contains: cobalt, nickel & cadmium) & kills most plants, but some species of plants can survive soil made with serpentine rock.

Concept 2. 2 An Element’s Properties Depend on the Structure of its Atoms Atom = smallest building block of matter still retaining the properties of the element it belongs to. Subatomic Particles protons, electrons & neutrons nucleus contains protons & neutrons mass of p+ & no are 1. 7 x 10 -24 grams = 1 Dalton or 1 amu o Electron’s mass is 1/2000 Daltons

o Named after British chemist, John Dalton, who helped develop the atomic theory

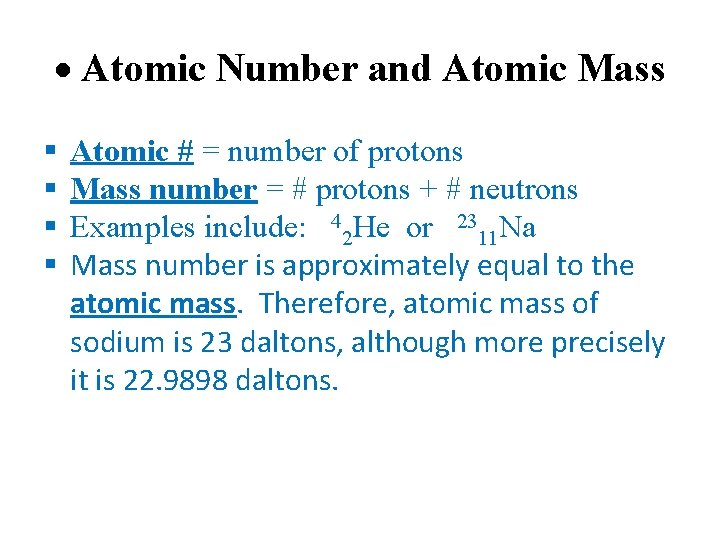
Atomic Number and Atomic Mass Atomic # = number of protons Mass number = # protons + # neutrons Examples include: 42 He or 2311 Na Mass number is approximately equal to the atomic mass. Therefore, atomic mass of sodium is 23 daltons, although more precisely it is 22. 9898 daltons.

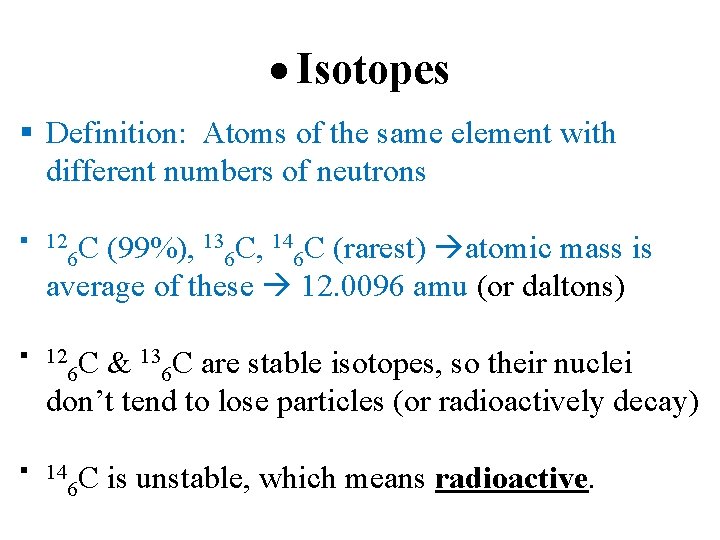
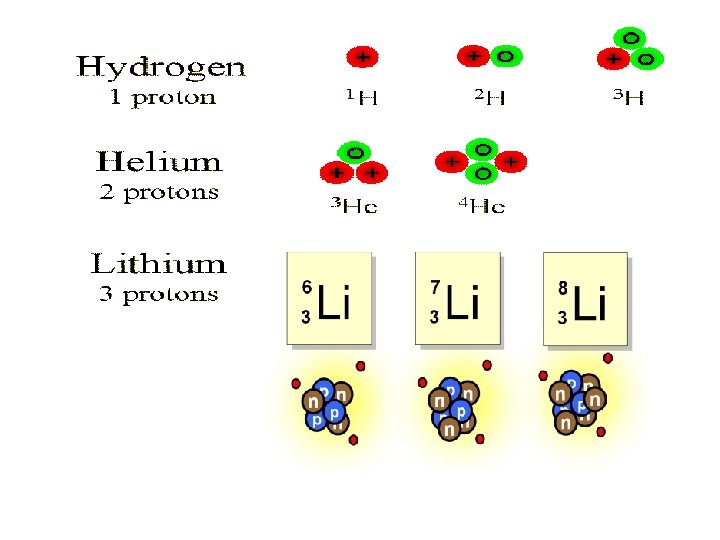
Isotopes Definition: Atoms of the same element with different numbers of neutrons 12 C 6 (99%), 136 C, 146 C (rarest) atomic mass is average of these 12. 0096 amu (or daltons) 12 C 6 & 136 C are stable isotopes, so their nuclei don’t tend to lose particles (or radioactively decay) 14 C 6 is unstable, which means radioactive.


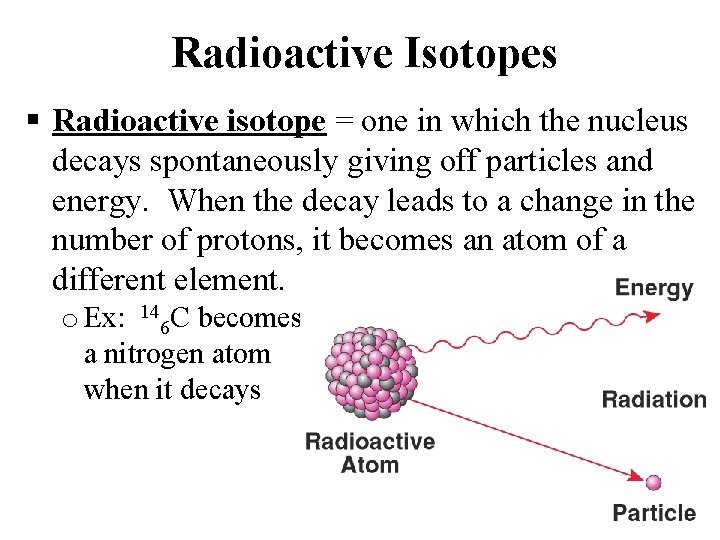
Radioactive Isotopes Radioactive isotope = one in which the nucleus decays spontaneously giving off particles and energy. When the decay leads to a change in the number of protons, it becomes an atom of a different element. o Ex: 146 C becomes a nitrogen atom when it decays

Radioactive Decay: Half-life • Bozeman: Radiocarbon Dating • Carbon Dating 100% Accurate Right? …Not • Go to the Khan Academy link below: www. khanacademy. org/science/chemistry/nucl ear-chemistry/radioactive-decay/v/half-life • How much time would have occurred if….

Continued… • Radioactive isotopes have uses: o Dating fossils o Serving as tracers to follow them through the metabolic pathway. Ex: Diagnose some kidney disorders by being injected in small doses into the blood & then measuring the amount of tracer excreted in the urine. o Used in PET Scans


Also used in PET scans.

o Although they can be useful to living things, they can also be dangerous to life by damaging cellular molecules. Ex: those given off by nuclear power plant accidents.

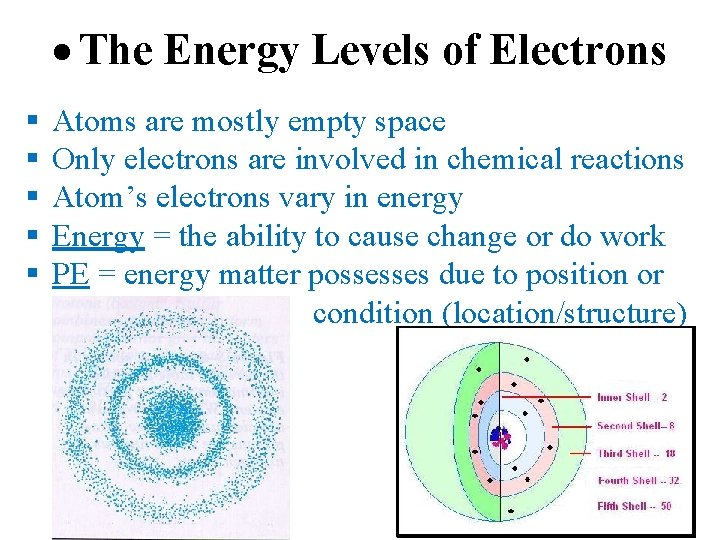
The Energy Levels of Electrons Atoms are mostly empty space Only electrons are involved in chemical reactions Atom’s electrons vary in energy Energy = the ability to cause change or do work PE = energy matter possesses due to position or condition (location/structure)

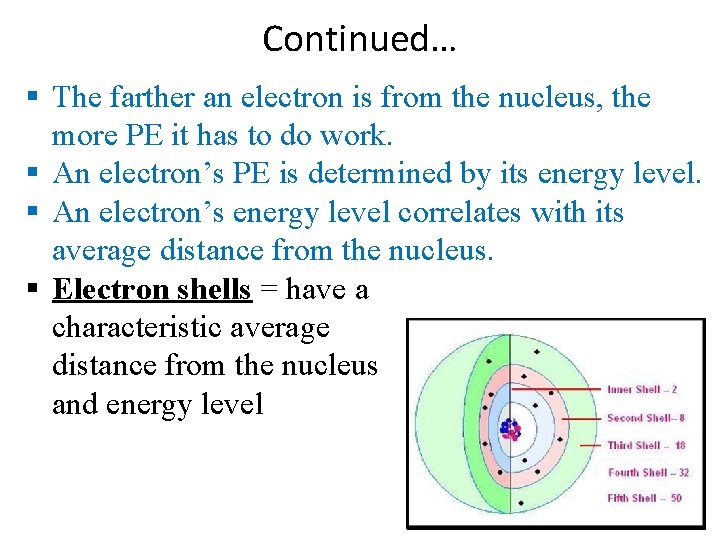
Continued… The farther an electron is from the nucleus, the more PE it has to do work. An electron’s PE is determined by its energy level. An electron’s energy level correlates with its average distance from the nucleus. Electron shells = have a characteristic average distance from the nucleus and energy level

Continued… An electron can change the shell it occupies, but only by absorbing or losing an amount of energy equal to the difference in PE between its position in the old shell and that in the new shell. When an electron absorbs energy, it moves to a shell further out from the nucleus. Ex: light energy can excite an electron to a higher energy level (this is the first step in photosynthesis).

Continued… Vice versa, an electron moves to a closer shell when it loses energy, that lost energy is usually given off as heat. o Sunlight can excite electrons on a car’s surface. When the electron loses energy and falls back to original energy levels, the energy is released as heat and the car’s surface heats up.

• Electron Distribution & Chemical Properties Electron distribution in the electron shells determines the chemical properties of the atom The chemical behavior of an atom depends mostly on the number of electrons in its outermost shell. These are called valence electrons & the outermost shell is called the valence shell.


Lesson 2: Chemical Bond-Forming Molecules • You. Tube Videos o Bozeman “Covalent vs. Ionic” “Ionic and Covalent Bonding Animation” o Biology Crash Course “Polar vs. Nonpolar”

Chemical Bonding • Chemical bonds are formed when atoms interact with each other to complete their valence shells. This is done by gaining, losing or sharing electrons. Covalent Bonds = form when pairs of electrons are shared. Ionic Bonds = attraction between oppositely charged atoms.

Covalent Bonds • Molecules = consist of 2 or more covalently bonded atoms. • Electronegativity = the attraction of an atom for the shared electrons. The more electronegative, the more attraction. o Oxygen is the 2 nd most electronegative atom (has 2 nd highest electronegativity).

Polar vs. Nonpolar Covalent Bonds • Polar Covalent Bonds = result from one atom being more electronegative than the other atom, with which it shares electrons. Atoms share the electrons unequally. These are weaker than nonpolar, but stronger than all other types of bonds. • Nonpolar Covalent Bonds = result from atoms, with equal electronegativity sharing atoms. Atoms share electrons equally. These are the strongest bonds.

Ionic Bonds Ion = forms when an atom or molecule gains or loses 1 or more electrons and becomes charged. Ionic Bond = the attraction between 2 oppositely charged electrons o Weaker than covalent bonds, but stronger than intermolecular bonds (or forces) like Hydrogen bonds.

Lesson 3: Intermolecular Forces You. Tube Video: • Dipole Forces (ex: Hydrogen & Van der Waals) • Dipole-dipole forces = Weak, temporary bonds that form between atom of one molecule and an atom in another molecule or between atoms in the same macromolecule, as in proteins. Can’t form new molecules or compounds Ex: Hydrogen bonds, Van der Waals interactions

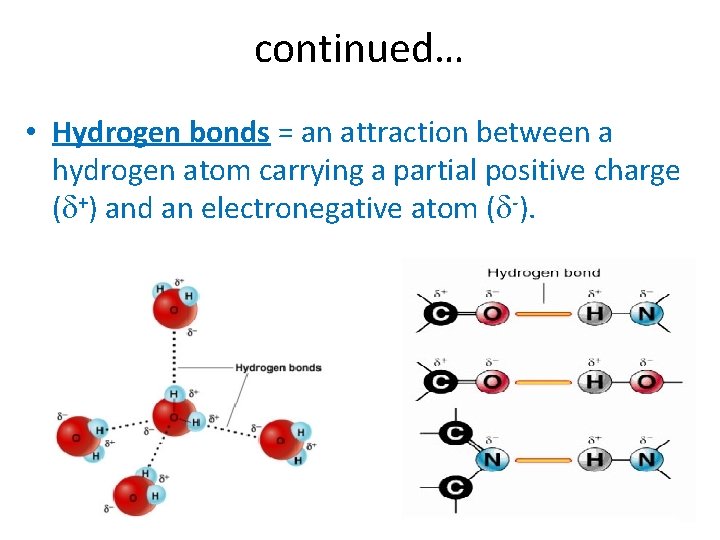
continued… • Hydrogen bonds = an attraction between a hydrogen atom carrying a partial positive charge (d+) and an electronegative atom (d-).

continued… • Van der Waals Interations = occur between transient positive regions and transient negative regions. Go to Simulation and choose “Run”.

Lesson 4: Water & its Properties • Watch You. Tube Video by Bozeman o Water and Life • Four Emergent Properties of Water 1) 2) 3) 4) Cohesive Behavior High Heat Capacity Expands upon Freezing Versatility as a Solvent

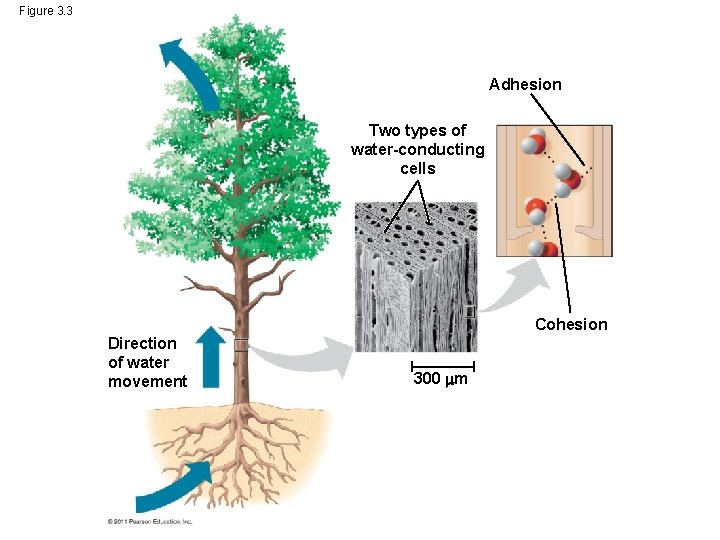
continued… • Cohesive Behavior o cohesion = water molecules held together by forming hydrogen bonds causes water transport up a plant surface tension = a measure of how difficult it is to stretch or break the surface of a liquid. o adhesion = clinging of one substance to another. water molecules stick to other substances

Figure 3. 3 Adhesion Two types of water-conducting cells Cohesion Direction of water movement 300 m

Figure 3. 4

continued… • High Heat Capacity o Can absorb a lot of heat before increasing temperature even 1 degree o Specific Heat = the amount of heat that must be absorbed or lost for 1 g of that substance to change its temperature by 1 o. C.

continued… • Expands upon Freezing Ice floats in liquid water because hydrogen bonds in ice are more “ordered, ” making ice less dense. Water reaches its greatest density at 4°C If ice sank, all bodies of water would eventually freeze solid, making life impossible on Earth.

continued… • Versatility as a Solvent Water’s polar property allows it to form hydration shells around charged molecules to separate them from other particles of matter, “dissolving” that substance. Go to Solvent Simulation and select Run.

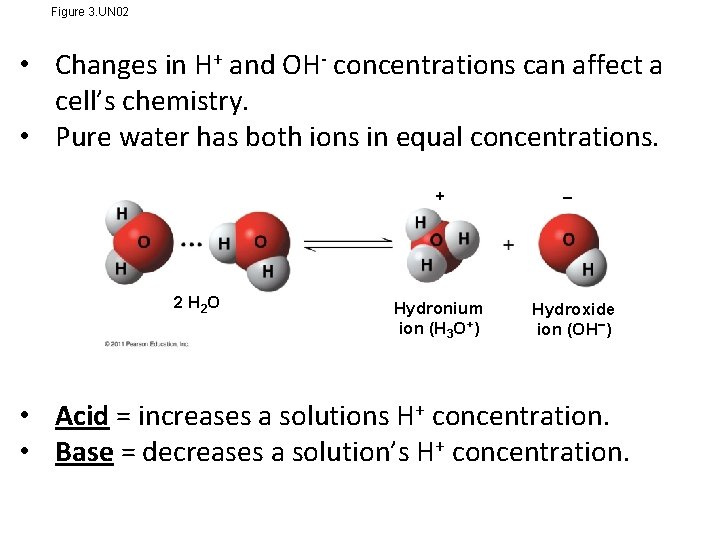
Lesson 5: Acidic & Basic Conditions • Hydrogen atoms in a hydrogen bond can shift from one water molecule to the other. • This causes a Hydrogen ion to form, which is unstable and will attach to the other water molecule forming a Hydronium ion (H 3 O+) and leaves the other water molecule as a hydroxide ion (OH-). • Go to p. H Simulation & select “Run”

Figure 3. UN 02 • Changes in H+ and OH- concentrations can affect a cell’s chemistry. • Pure water has both ions in equal concentrations. + 2 H 2 O Hydronium ion (H 3 O+) Hydroxide ion (OH ) • Acid = increases a solutions H+ concentration. • Base = decreases a solution’s H+ concentration.

continued… • Acids = p. H less than 7 • Bases = p. H greater than 7 • Most biological fluids have p. H values in the range of 6 to 8. Most living cells require p. H close to 7. • Buffers = consist of an acid-base pair that work together to minimize changes in H+ and OH-.

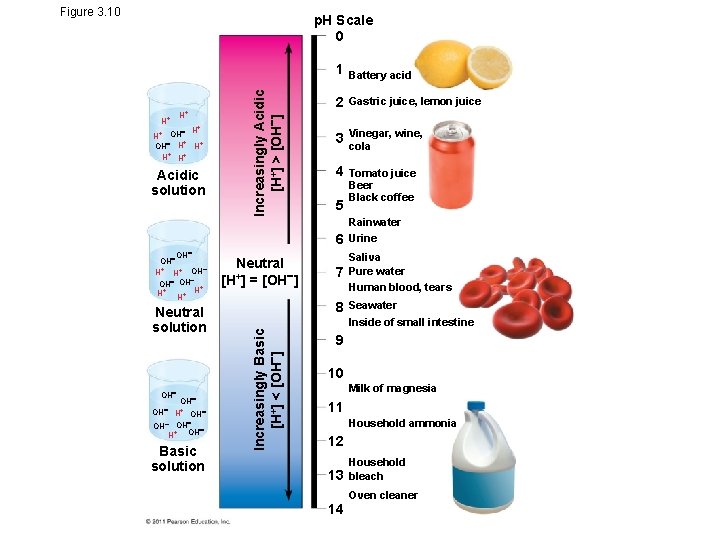
Figure 3. 10 H+ H+ + OH H H+ H+ Acidic solution Increasingly Acidic [H+] > [OH ] p. H Scale 0 1 Battery acid 2 Gastric juice, lemon juice 3 Vinegar, wine, cola 4 Tomato juice Beer Black coffee 5 6 OH H+ H+ OH OH + H+ H+ H Neutral solution OH OH H+ OH OH H+ OH Basic solution Neutral [H+] = [OH ] 7 8 Increasingly Basic [H+] < [OH ] OH Rainwater Urine Saliva Pure water Human blood, tears Seawater Inside of small intestine 9 10 Milk of magnesia 11 Household ammonia 12 13 14 Household bleach Oven cleaner

Acidification: A Threat to Water Quality CO 2 Acidification o CO 2 dissolved in sea water forms carbonic acid; this process is called ocean acidification o As seawater acidifies, H+ ions combine with carbonate ions to produce bicarbonate. o Carbonate is required for calcification (production of calcium carbonate) by many marine organisms, including reef-building corals.

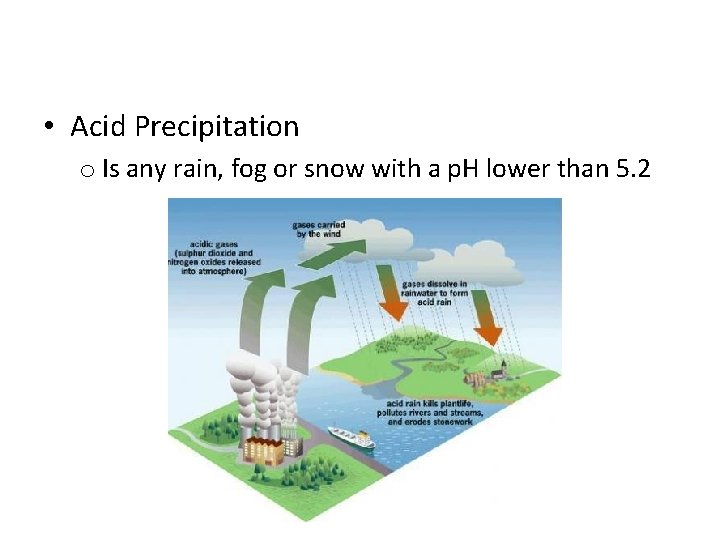
• Acid Precipitation o Is any rain, fog or snow with a p. H lower than 5. 2