Unit 1 Chemistry Chemistry the study of matter











































- Slides: 62

Unit 1 - Chemistry

Chemistry - the study of matter, its properties, and its changes or transformations. Matter - anything that has mass and takes up space. There is a need to classify matter. Let’s review how scientists classify matter.

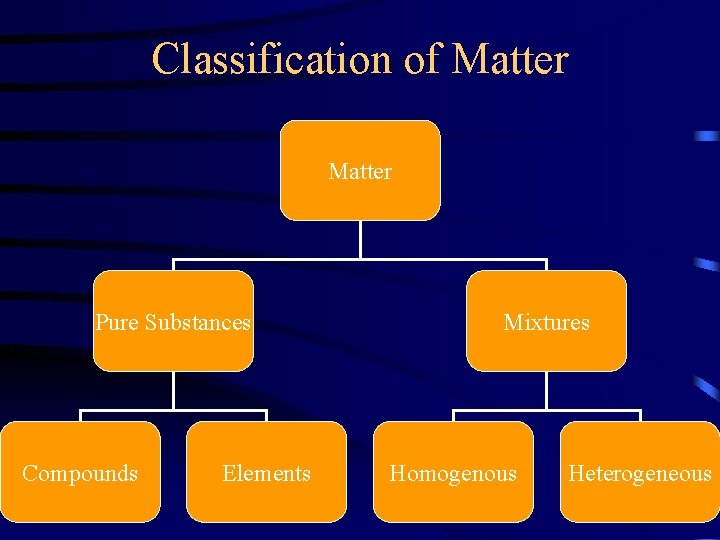
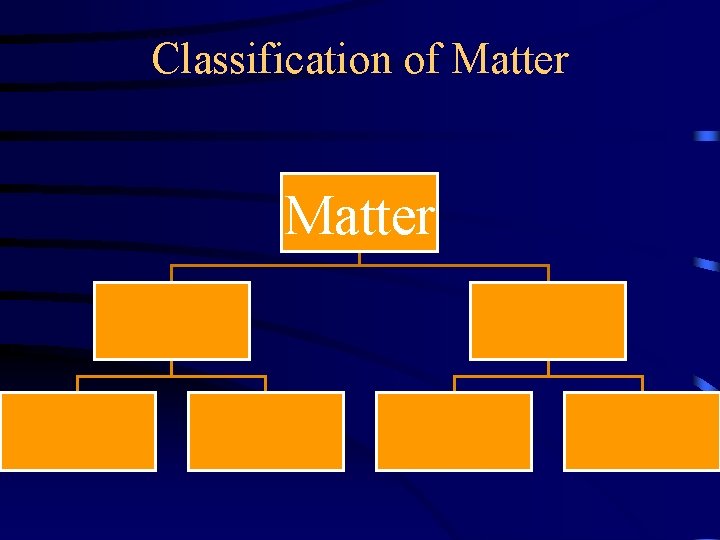
Classification of Matter Pure Substances Compounds Elements Mixtures Homogenous Heterogeneous

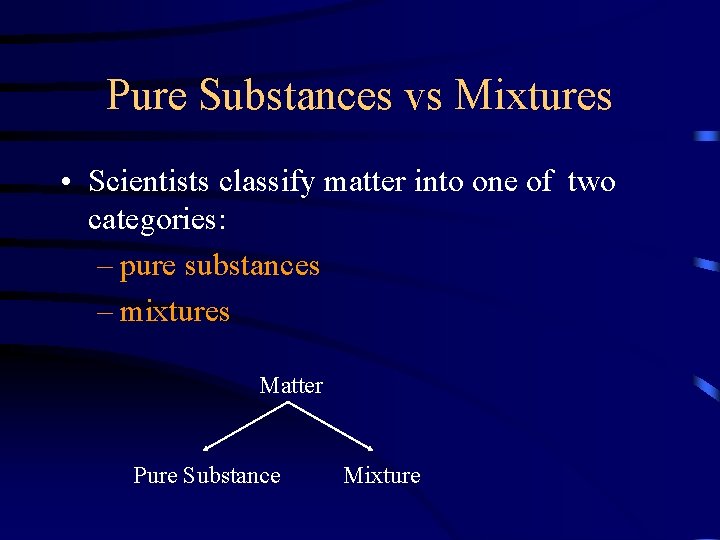
Pure Substances vs Mixtures • Scientists classify matter into one of two categories: – pure substances – mixtures Matter Pure Substance Mixture

Pure Substances • one in which all the particles that make up the substance are the same. • Examples include: – Copper wire contains only the metal copper. – sugar contains only sugar particles. • How are these two examples different though? – Copper is an element, sugar is made of a combination of several elements (carbon, hydrogen, and oxygen); a compound

Pure Substances • Chemists tend to classify pure substances on the basis of the particles of which they are made. – Pure substance are classified as either compounds or elements. Pure Substance Compounds Elements

Pure Substances Elements cannot be broken down into simpler substances. – Elements are the building blocks of matter!!! – All those elements on the periodic table.


• If you want to learn more about the elements online, check out these periodic tables: interactive 1 interactive 2 (click on)

Pure Substances • Compounds contain two or more different elements in a fixed proportion. – Examples: • water is H 2 O, (H: O = 2: 1) • carbon dioxide is CO 2, (C: O = 1: 2) • sugar is C 6 H 12 O 6 (C: H: O = 1: 2: 1) • In Science 9 you studied elements. In Science 10, we are going to focus on compounds, and chemical reactions.

Mixtures Contains at least two different pure substances. Examples: – Soft drink – Pizza – Sugar dissolved in water – Air – Water (not distilled) Mixtures are further classified:

How to Classify Mixtures When two pure substances are mixed together, they may mix smoothly or unevenly. • If the substances mix well with one another, the mixture is called a solution. – You cannot distinguish the parts. These are homogeneous mixtures. • If the particles don’t mix well together then we have a heterogeneous mixture.

• What would this be classified as?

• And this?

Classify the following mixtures previously cited. • Salt and water – Solution; homogenous mixture (looks like one thing) • Pizza – heterogeneous mixture (looks like many things) • Sugar dissolved in water – Solution; homogenous mixture (looks like one thing) • Air – Solution; homogenous mixture (looks like one thing)

Revision. Complete the following diagram with the correct term:

Classification of Matter

Revision. Complete the following diagram with an example of what belongs in each category:

Classification of Matter

Properties of Matter All matter has both physical and chemical properties.

Physical Properties A physical property is a characteristic of a substance. They include – The state of matter at room temperature – Hardness – Melting and boiling points – Odor – Color – Solubility, etc

Example: What are some of the physical properties of water? Water is a clear liquid at room temperature, which boils at 100 degrees celcius, freezes at 0 degrees celcius, and has a density of 1 g/cm 3 at SATP. We could of course say a lot more

Chemical Properties A chemical property describes the behavior of a substance as it becomes a new substance. – The change itself is called a chemical change. For example, when baking soda is added to an acid, a new substance, carbon dioxide gas, is formed. – This reaction with acid is a chemical property of baking soda.

Rusting is another chemical change that involves a metal and oxygen - a new substance, iron (III) oxide, or rust is formed when an iron reacts with oxygen Rusting is also called oxidation

Combustibility is another chemical property that describes the ability of a substance to react with oxygen to produce carbon dioxide, water and energy. - If a substance is combustible or flammable, it will burn when exposed to a flame. - A substance that will not burn is described as nonflammable. The starting materials in such a change are called the reactants. The resulting substances are called the products. Hydrocarbon + oxygen => carbon dioxide + water reactants products

Clues That a Chemical Change Has Happened • • A new color appears. Heat or light is given off. Bubbles of gas are formed. A solid material (called a precipitate) forms in a liquid. • The change is difficult to reverse.

Chemical Tests • Chemical changes can be useful to identify unknown substances. • For example, a geologist can add an acid to an unknown sample of rock. If bubbles of CO 2 are formed, the rock is probably limestone. • Some other examples of chemical testing are outlined on the following slide.

Chemical Tests - examples Oxygen gas is indicated if a glowing splint (amber) burst into flames when placed in the gas Hydrogen gas is indicated if a flaming splint at the mouth of the test tube causes a “pop” or a small explosion. Carbon dioxide gas is indicated if limewater turns milky when the gas is bubbled into it.

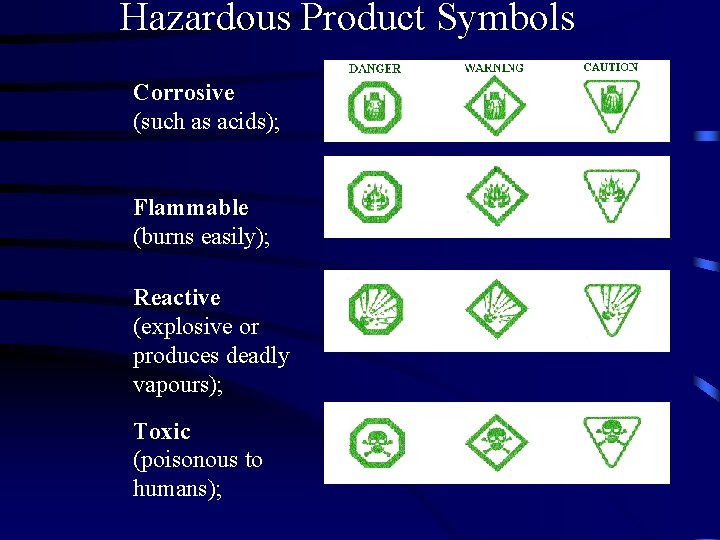
Hazardous Product Symbols Corrosive (such as acids); Flammable (burns easily); Reactive (explosive or produces deadly vapours); Toxic (poisonous to humans);

The following slides are really a review of what you covered in grade 9 again! I would therefore recommend that you just pay attention or write very brief notes as each slide is discussed. The presentation is found on Edline.

Organizing the Elements Up to the mid-1800 s, scientists were busy discovering new elements. • Then they tried to organize the elements alphabetically. But every time a new element was discovered, the whole list had to be changed.

Organizing the Elements Other methods of organization were considered but later discarded. – State (gas, solid, liquid) – Color – Taste John Dalton then found a quantity that could be measured for an element - its atomic mass. – Several scientists then tried to arrange the known elements by their atomic masses. • The best arrangement was produced by a Russian scientist, Dmitri Mendeleev.

Mendeleev began arranging the elements in order of increasing atomic mass and noticed that many elements shared common properties. - These elements typically belonged to the same vertical column of his table. – arrangement showed a regular pattern. Mendeleev’s periodic law states: “If the elements are arranged according to their atomic mass, a pattern can be seen in which similar properties occur regularly. ”

Mendeleev’s periodic table was a major breakthrough in the understanding of the elements. However, it was discovered later on that using the atomic mass was not the proper way to organize the elements. – The key was to use the atomic number or the number of protons. Therefore, a new law was born. – The modern periodic law states : If the elements are arranged according to their atomic number, a pattern can be seen in which similar properties occur regularly.

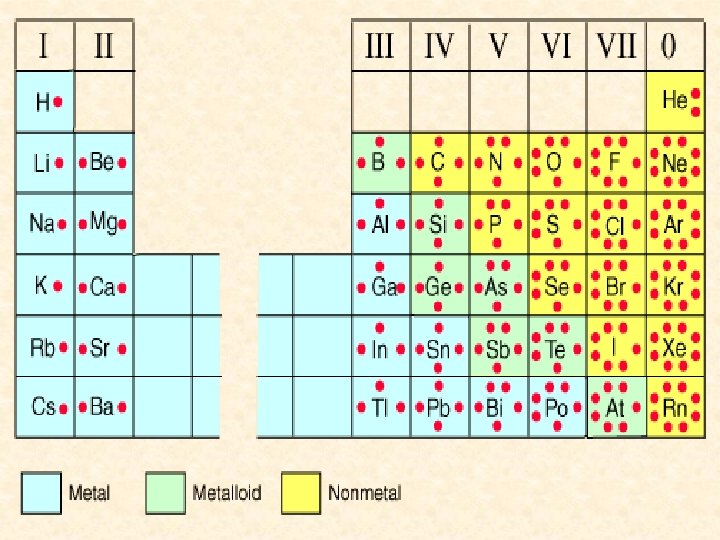
Organizing the Elements All the elements in a column on the table are called a chemical group or family. – have similar physical and chemical properties. • form similar kinds of compounds when they combine with other elements.

Organizing the Elements Alkali Metals • The first column of elements • are all shiny, silvery metals that are extremely reactive. • very soluble in water. • Because they combine so easily with other elements, they are only found in nature in the form of compounds. • Examples include lithium, sodium and potassium.

Organizing the Elements The Alkaline Earth Metals • The second column • also shiny silvery metals but they form compounds that are often insoluble in water. • include magnesium (Mg), calcium (Ca), and barium (Ba).

Organizing the Elements Metalloids • have both metallic and nonmetallic properties • make up the staircase on the periodic table. • silicon (Si), boron (B), and antimony (Sb).

Organizing the Elements Halogens • 17 th column on the periodic table. • poisonous elements that react readily with sodium and other alkali metals. Ex. Na. Cl or salt • are the most reactive non-metals and they almost always appear in nature in the form of a compound.

Organizing the Elements The Noble Gases • The last column • unreactive and almost never form compounds with other elements. Why are they stable? ? Hydrogen • in a group of its own because it is unique • has the ability to react like a metal as well as a non-metal … forms ionic and covalent bonds!

Organizing the Elements Rows on the Periodic Table • horizontal rows are called periods. • As you move from left to right in a row the atomic number increases and the elements change from metals to nonmetals to the noble gases … the staircase dividing the metals from the non-metals.

Models of Matter • 1911 – Ernest Rutherford, working in Montreal developed another revised model, the nuclear model: – Atoms have a tiny, dense, positive core called a nucleus. – The nucleus is surrounded mostly by empty space, containing rapidly moving negative electrons.

Models of Matter • A Danish physicist, Niels Bohr, proposed a “planetary” model of the atom. – Electrons move around the nucleus in a circular path called orbits. – The farther away the electron is from the nucleus, the greater its energy. – Electrons cannot exist between these orbits, but can move from one orbit to another. – The order of filling of electrons in the first three orbits is 2, 8, and 8. – DID NOT SHOW PROTONS OR NEUTRONS

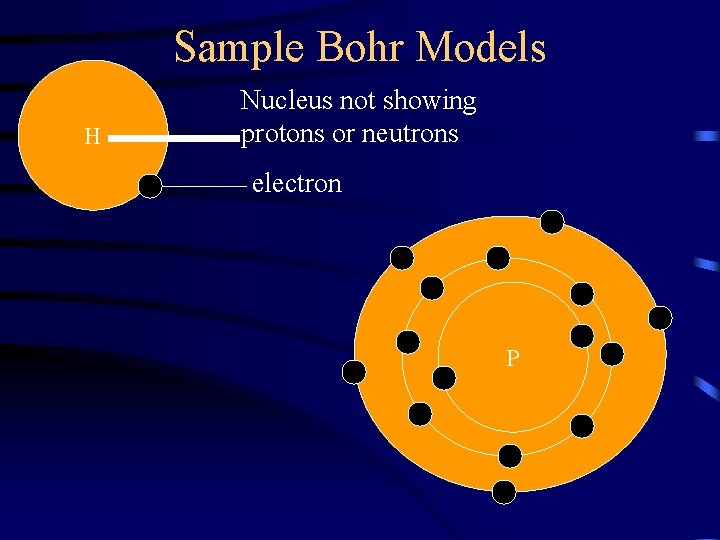
Sample Bohr Models H Nucleus not showing protons or neutrons electron P

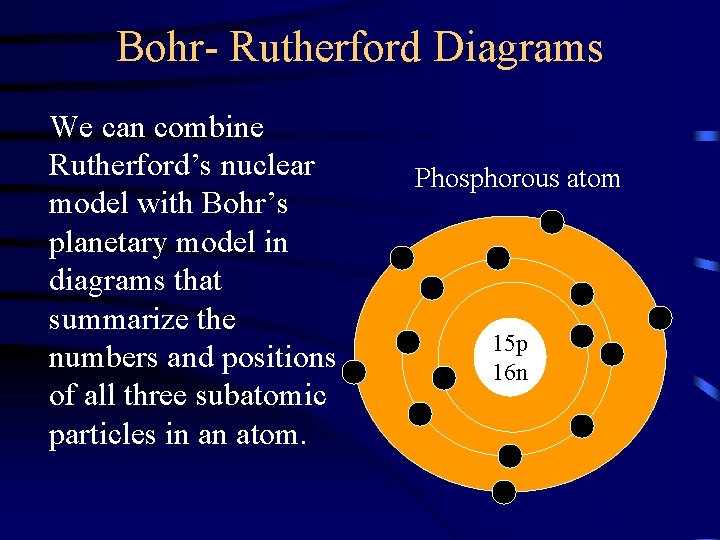
Bohr- Rutherford Diagrams We can combine Rutherford’s nuclear model with Bohr’s planetary model in diagrams that summarize the numbers and positions of all three subatomic particles in an atom. Phosphorous atom 15 p 16 n

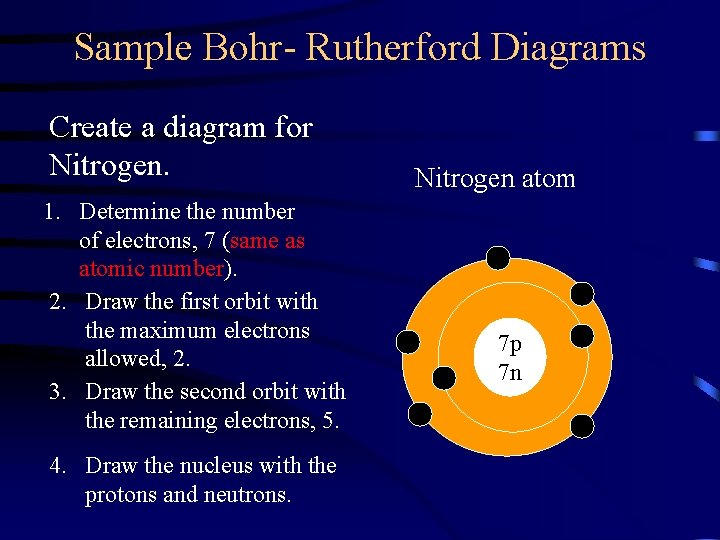
Sample Bohr- Rutherford Diagrams Create a diagram for Nitrogen. 1. Determine the number of electrons, 7 (same as atomic number). 2. Draw the first orbit with the maximum electrons allowed, 2. 3. Draw the second orbit with the remaining electrons, 5. 4. Draw the nucleus with the protons and neutrons. Nitrogen atom 7 p 7 n

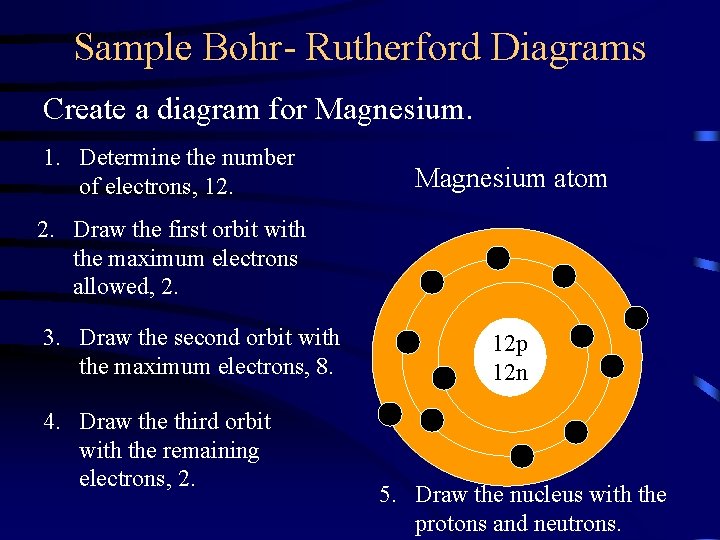
Sample Bohr- Rutherford Diagrams Create a diagram for Magnesium. 1. Determine the number of electrons, 12. Magnesium atom 2. Draw the first orbit with the maximum electrons allowed, 2. 3. Draw the second orbit with the maximum electrons, 8. 4. Draw the third orbit with the remaining electrons, 2. 12 p 12 n 5. Draw the nucleus with the protons and neutrons.

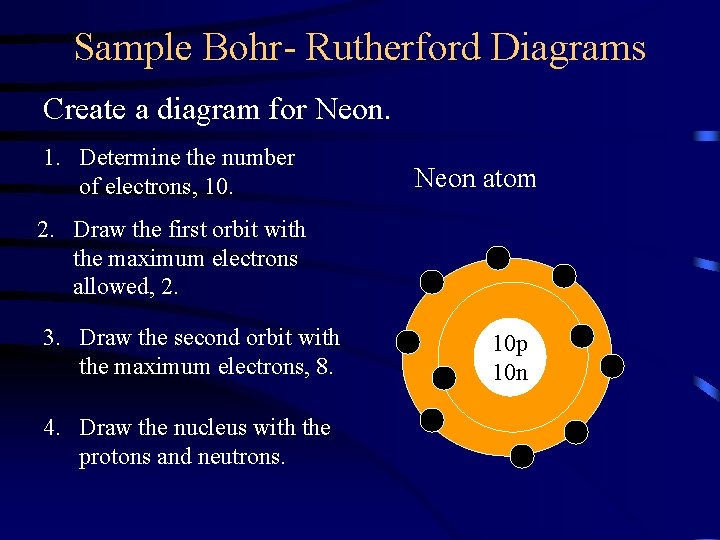
Sample Bohr- Rutherford Diagrams Create a diagram for Neon. 1. Determine the number of electrons, 10. Neon atom 2. Draw the first orbit with the maximum electrons allowed, 2. 3. Draw the second orbit with the maximum electrons, 8. 4. Draw the nucleus with the protons and neutrons. 10 p 10 n

Inside the Atom • We have spent a great deal of time discussing atoms but what do they look like? • Atoms are “particles” that are comprised of smaller particles called “subatomic particles”. – Electrons, protons, and neutrons are subatomic particles.

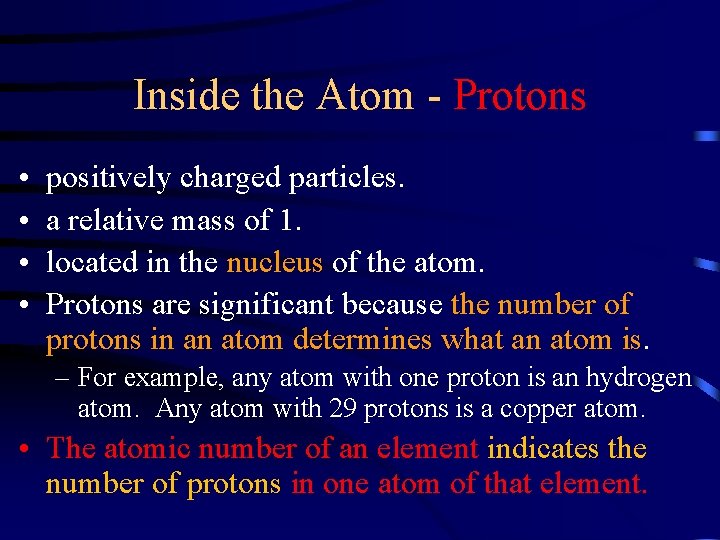
Inside the Atom - Protons • • positively charged particles. a relative mass of 1. located in the nucleus of the atom. Protons are significant because the number of protons in an atom determines what an atom is. – For example, any atom with one proton is an hydrogen atom. Any atom with 29 protons is a copper atom. • The atomic number of an element indicates the number of protons in one atom of that element.

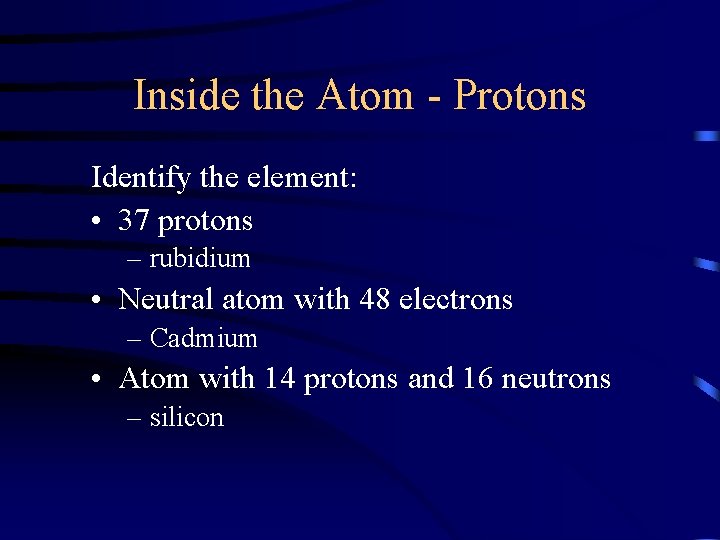
Inside the Atom - Protons Identify the element: • 37 protons – rubidium • Neutral atom with 48 electrons – Cadmium • Atom with 14 protons and 16 neutrons – silicon

Inside the Atom- Electrons • negatively charged particles. • have a relative mass of 1/2000 of a proton. – And therefore don’t really contribute to the mass of an atom. • travel in regions of space around the nucleus of the atom (orbit or energy level).

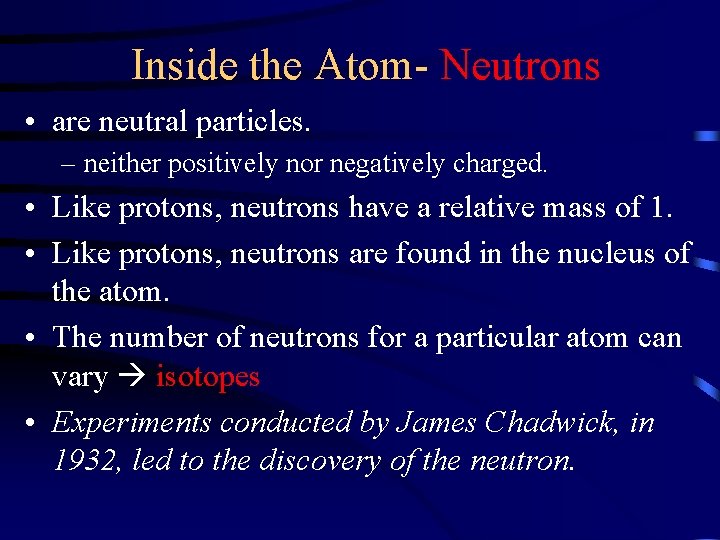
Inside the Atom- Neutrons • are neutral particles. – neither positively nor negatively charged. • Like protons, neutrons have a relative mass of 1. • Like protons, neutrons are found in the nucleus of the atom. • The number of neutrons for a particular atom can vary isotopes • Experiments conducted by James Chadwick, in 1932, led to the discovery of the neutron.

Atomic Number • The atomic number tells us the number of protons in one atom of the element. • The atomic number also tells us the number of electrons in one atom of (the neutral) element. – The number of electrons is equal to the number of protons. Example: the atomic number of calcium is 20 therefore the number of protons is 20 and the number of electrons is also 20.

The Atomic Mass • The atomic mass tells us the total number of protons and neutrons in one atom of the element. – Therefore, knowing the atomic number and the atomic mass we can determine the number of neutrons. # of neutrons = atomic mass - atomic number Example: the atomic number of calcium is 20 and the atomic mass is 40. Therefore, the number of neutrons is 40 -20 = 20.

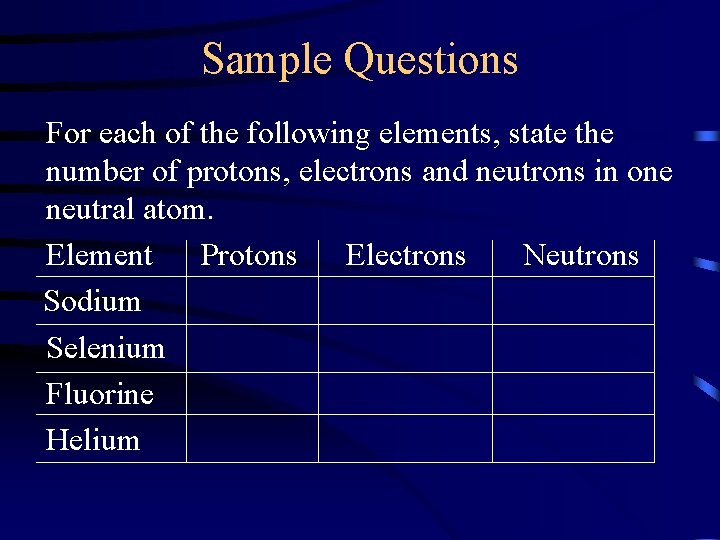
Sample Questions For each of the following elements, state the number of protons, electrons and neutrons in one neutral atom. Element Protons Electrons Neutrons Sodium Selenium Fluorine Helium

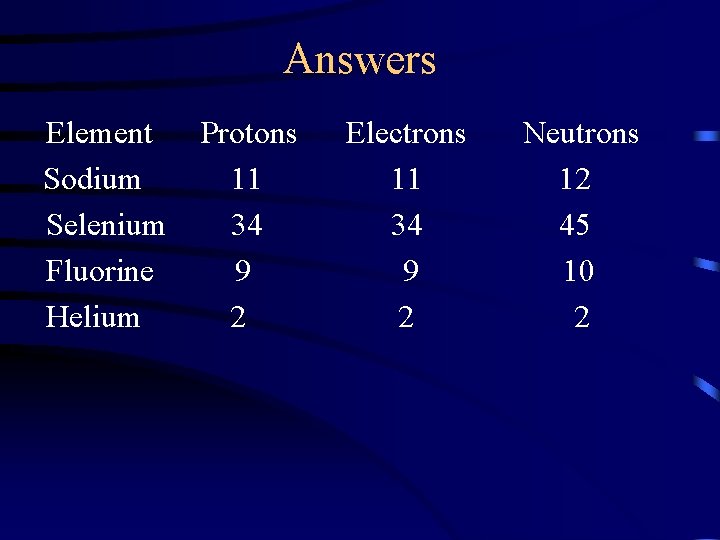
Answers Element Sodium Selenium Fluorine Helium Protons 11 34 9 2 Electrons 11 34 9 2 Neutrons 12 45 10 2

Lewis Structures of atoms Or Electron Dot Diagrams

Lewis Structures of atoms • The Lewis structure for an individual atom is drawn by placing a dot around the atom for each valence electron available. • Valence electrons refer to the number of electrons in the outer orbit or shell of an atom. • There are four positions available for dots to be placed; top, left, bottom, and right of the symbol for the atom. • Each of the four positions must receive one electron (one dot) before any electrons can be paired with one another. (Helium is an exception; the two electrons are shown as a pair. )


HW Copy the following down, in case you don’t get through all the notes today. These are due tomorrow. • Read 172 – 174 and do questions 1 - 9 • Read section 5. 5, on pages 184 -87, and do questions 1 – 4. These are questions similar to what you did in grade 9 science. don’t start now …watch the

HW Alright, you can now start the homework. Enjoy! • Read 172 – 174 and do questions 1 - 9 • Read section 5. 5, on pages 184 -87, and do questions 1 – 4. These are questions similar to what you did in grade 9 science.