UNIT 1 BIOCHEMISTRY Part 2 Hillis Textbook Chapter
UNIT 1 BIOCHEMISTRY Part 2 Hillis Textbook Chapter 2 -3 Chem Properties 2007 -2008
This week: • Monday: chemistry vocabulary and wrap-up • Tuesday: Biomolecules, hydrolysis and dehydration synthesis. Homework reactions/metabolism • Wednesday: Quiz of metabolism, Biomolecule card sort and chart completion for a grade! Pre-Lab handout for homework! • Thursday: LAB -TESTING FOR BIOMOLECULES! Practice using indicators to test for the presence in foods. • Friday: Share out with the class – lab data and results Next Week: • Monday: Enzyme lecture Pre-Lab for homework • Tuesday: LAB – ENZYME ACTION OF CATALASE • Wednesday: Share out with the class – lab data and results. Homework study for exam and finish objectives • Thursday: Exam 1 multiple choice and grid • Friday: Exam 1 free response portion
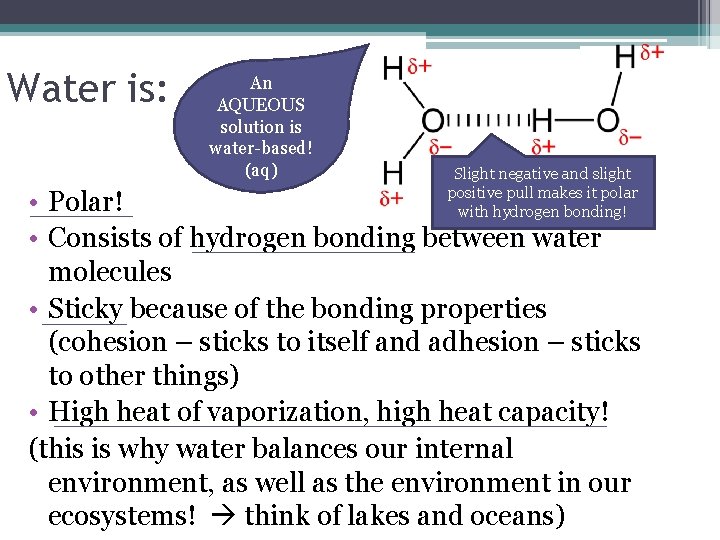
Water is: An AQUEOUS solution is water-based! (aq) Slight negative and slight positive pull makes it polar with hydrogen bonding! • Polar! • Consists of hydrogen bonding between water molecules • Sticky because of the bonding properties (cohesion – sticks to itself and adhesion – sticks to other things) • High heat of vaporization, high heat capacity! (this is why water balances our internal environment, as well as the environment in our ecosystems! think of lakes and oceans)

HYDROPHILIC vs. HYDROPHOBIC • Hydrophilic – having an affinity to water and capable of interacting with water through hydrogen bonding. ▫ Look for polar groups… • Hydrophobic – tendency for non-polar substances to aggregate in an aqueous solution and exclude water. ▫ Look for non polar groups… • Amphipathic/Amphiphiles – molecules with hydrophilic and hydrophobic domains. ▫ When interacting with water, the polar ends will face out and non-polar ends will face in, to form a bilayer or a micelle. ▫ Examples include detergents (soap bubbles) and the cell membrane.
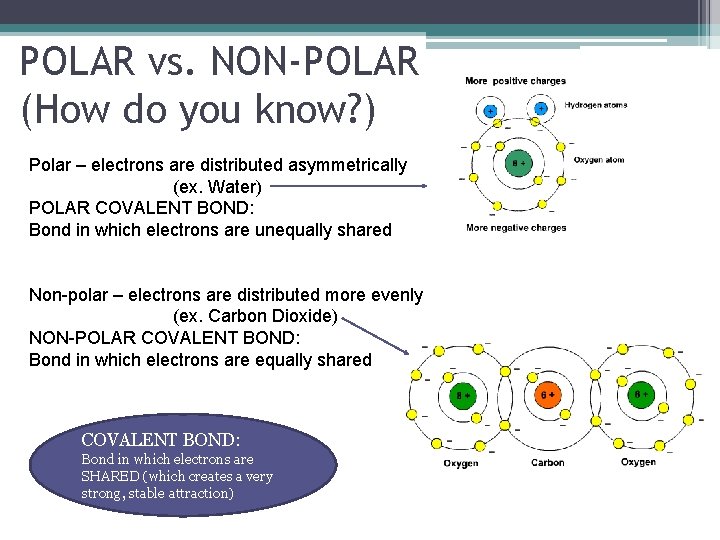
POLAR vs. NON-POLAR (How do you know? ) Polar – electrons are distributed asymmetrically (ex. Water) POLAR COVALENT BOND: Bond in which electrons are unequally shared Non-polar – electrons are distributed more evenly (ex. Carbon Dioxide) NON-POLAR COVALENT BOND: Bond in which electrons are equally shared COVALENT BOND: Bond in which electrons are SHARED (which creates a very strong, stable attraction)
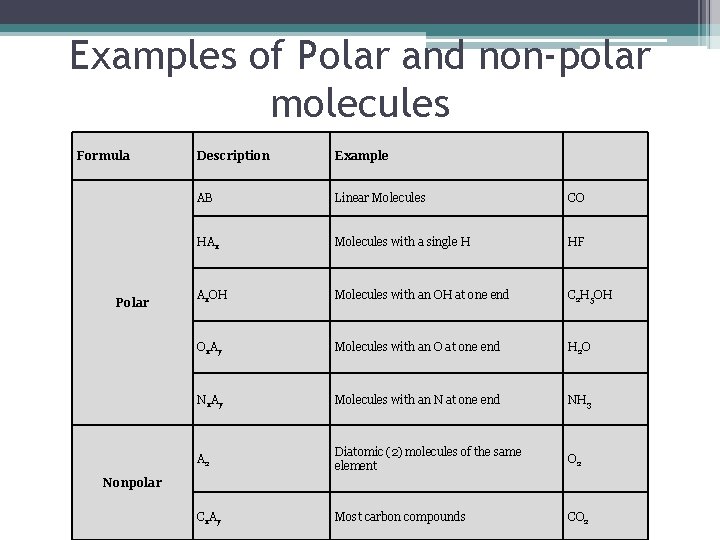
Examples of Polar and non-polar molecules Formula Polar Description Example AB Linear Molecules CO HAx Molecules with a single H HF Ax. OH Molecules with an OH at one end C 2 H 5 OH Ox. Ay Molecules with an O at one end H 2 O Nx. Ay Molecules with an N at one end NH 3 A 2 Diatomic (2) molecules of the same element O 2 Cx. Ay Most carbon compounds CO 2 Nonpolar
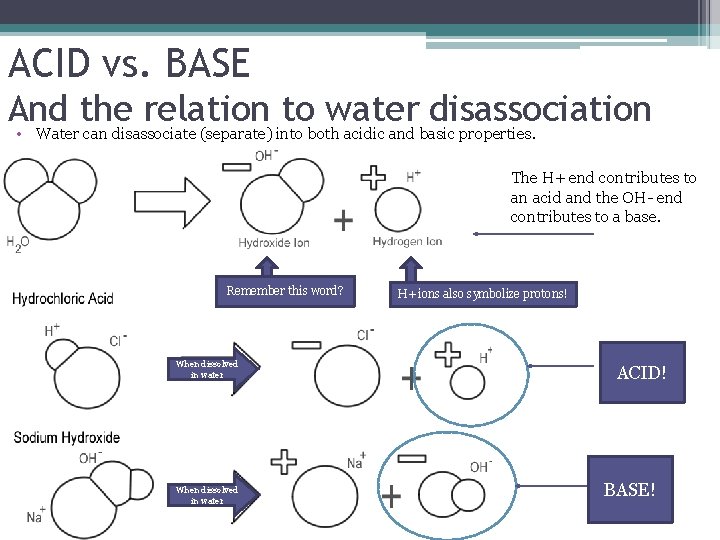
ACID vs. BASE And the relation to water disassociation • Water can disassociate (separate) into both acidic and basic properties. The H+ end contributes to an acid and the OH- end contributes to a base. Remember this word? When dissolved in water H+ ions also symbolize protons! ACID! BASE!
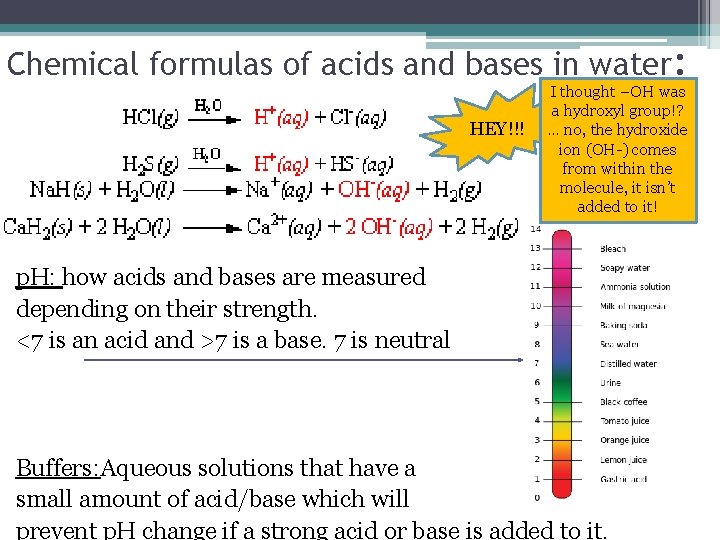
Chemical formulas of acids and bases in water: HEY!!! I thought –OH was a hydroxyl group!? … no, the hydroxide ion (OH-) comes from within the molecule, it isn’t added to it! p. H: how acids and bases are measured depending on their strength. <7 is an acid and >7 is a base. 7 is neutral Buffers: Aqueous solutions that have a small amount of acid/base which will prevent p. H change if a strong acid or base is added to it.
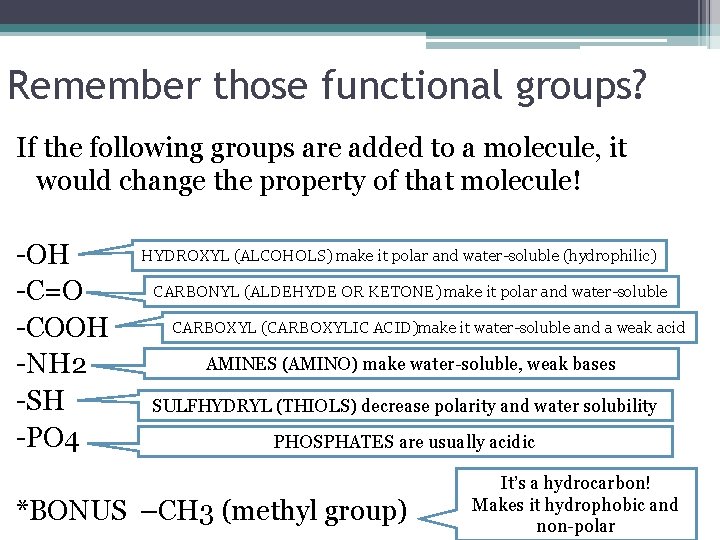
Remember those functional groups? If the following groups are added to a molecule, it would change the property of that molecule! -OH -C=O -COOH -NH 2 -SH -PO 4 HYDROXYL (ALCOHOLS) make it polar and water-soluble (hydrophilic) CARBONYL (ALDEHYDE OR KETONE) make it polar and water-soluble CARBOXYL (CARBOXYLIC ACID)make it water-soluble and a weak acid AMINES (AMINO) make water-soluble, weak bases SULFHYDRYL (THIOLS) decrease polarity and water solubility PHOSPHATES are usually acidic *BONUS –CH 3 (methyl group) It’s a hydrocarbon! Makes it hydrophobic and non-polar

WORK ON YOUR OBJECTIVES! • OBJECTIVES ARE DUE SEPT 19 th • WE HAVE ALREADY COVERED 9 of the 18. … YES, THAT IS HALF. P. S. let’s start practicing classroom procedures. I DO NOT like you out of your seat until the bell rings, unless otherwise instructed. THANK YOU!!!!!
UNIT 1 BIOCHEMISTRY Part 2 Hillis Textbook Chapter 2 -3 Chem Properties 2007 -2008
This week: • Monday: chemistry vocabulary and wrap-up • Tuesday: Biomolecules, hydrolysis and dehydration synthesis. Homework reactions/metabolism • Wednesday: Quiz of metabolism, Biomolecule card sort and chart completion for a grade! Pre-Lab handout for homework! • Thursday: LAB -TESTING FOR BIOMOLECULES! Practice using indicators to test for the presence in foods. • Friday: Share out with the class – lab data and results Next Week: • Monday: Enzyme lecture Pre-Lab for homework • Tuesday: LAB – ENZYME ACTION OF CATALASE • Wednesday: Share out with the class – lab data and results. Homework study for exam and finish objectives • Thursday: Exam 1 multiple choice and grid • Friday: Exam 1 free response portion

Water is: • _________ • Consists of _____________ • Sticky because of the bonding properties (_________________) • __________________ (this is why water balances our internal environment, as well as the environment in our ecosystems! think of lakes and oceans)
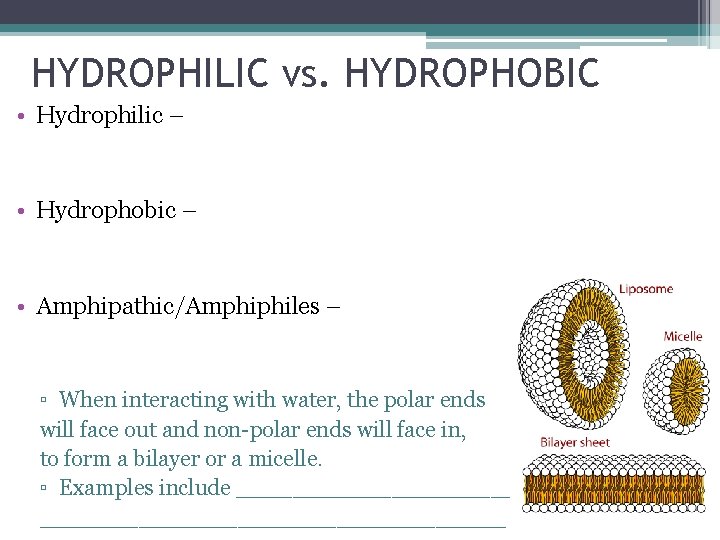
HYDROPHILIC vs. HYDROPHOBIC • Hydrophilic – • Hydrophobic – • Amphipathic/Amphiphiles – ▫ When interacting with water, the polar ends will face out and non-polar ends will face in, to form a bilayer or a micelle. ▫ Examples include ___________________________
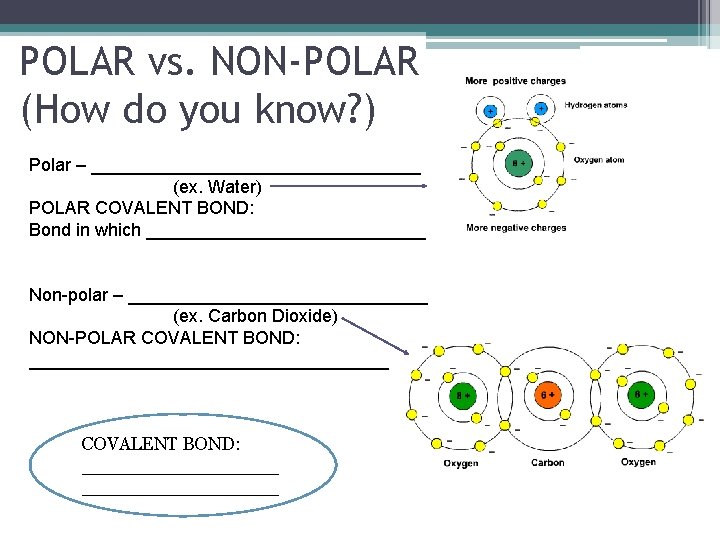
POLAR vs. NON-POLAR (How do you know? ) Polar – _________________ (ex. Water) POLAR COVALENT BOND: Bond in which ______________ Non-polar – _______________ (ex. Carbon Dioxide) NON-POLAR COVALENT BOND: __________________ COVALENT BOND: _________________
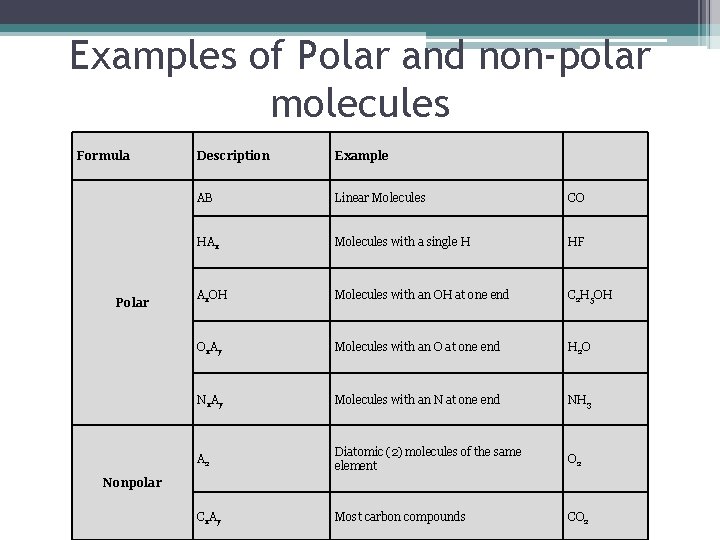
Examples of Polar and non-polar molecules Formula Polar Description Example AB Linear Molecules CO HAx Molecules with a single H HF Ax. OH Molecules with an OH at one end C 2 H 5 OH Ox. Ay Molecules with an O at one end H 2 O Nx. Ay Molecules with an N at one end NH 3 A 2 Diatomic (2) molecules of the same element O 2 Cx. Ay Most carbon compounds CO 2 Nonpolar
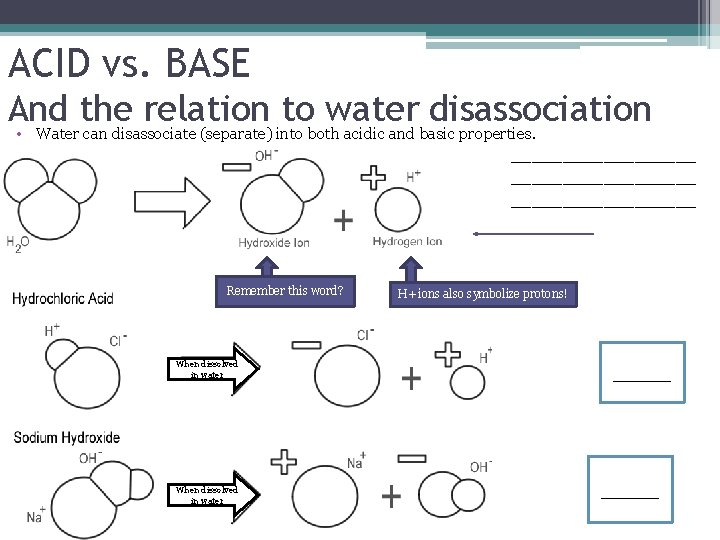
ACID vs. BASE And the relation to water disassociation • Water can disassociate (separate) into both acidic and basic properties. __________________ Remember this word? When dissolved in water H+ ions also symbolize protons! _____
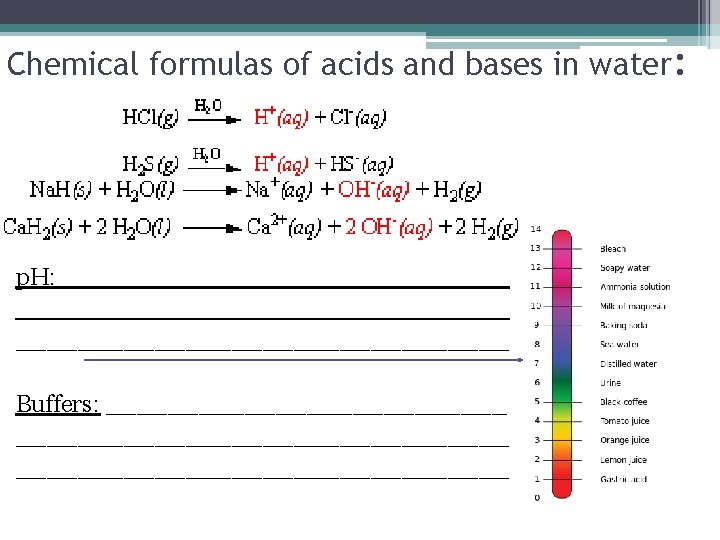
Chemical formulas of acids and bases in water: p. H: ________________________________ Buffers: ________________________________
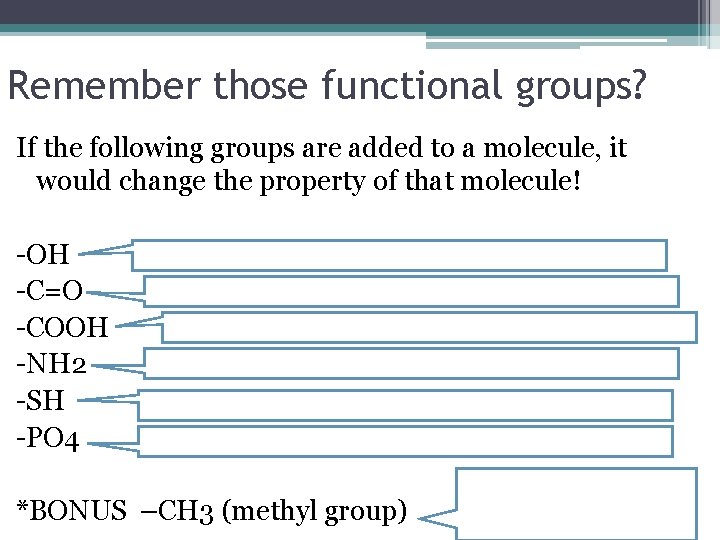
Remember those functional groups? If the following groups are added to a molecule, it would change the property of that molecule! -OH -C=O -COOH -NH 2 -SH -PO 4 *BONUS –CH 3 (methyl group)
MACROMOLECULES OF LIFE • BIOMOLECULES – macromolecules essential for living things to survive.

Macromolecules • Smaller organic molecules join together to form larger molecules ▫ macromolecules • 4 major classes of macromolecules: ▫ ▫ carbohydrates lipids proteins nucleic acids
Polymers and monomers Polymers: Long molecules built by linking repeating building blocks in a chain Monomers: building blocks of the polymers! • Small, repeated units • Made from covalent bonding!
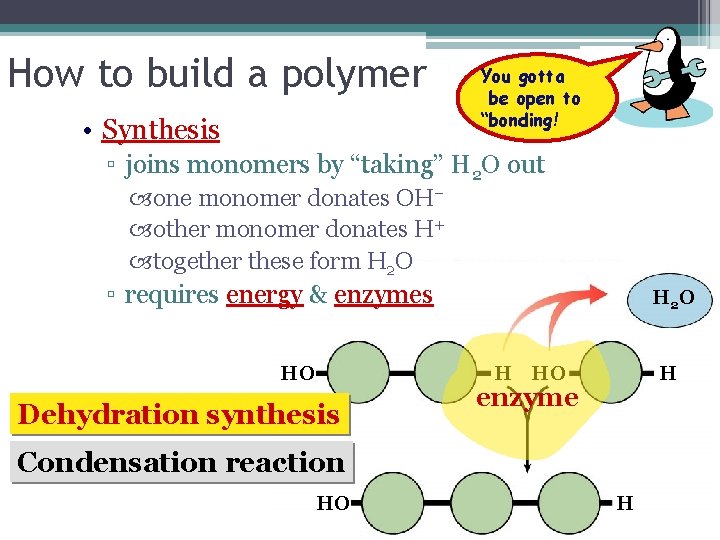
How to build a polymer • Synthesis You gotta be open to “bonding! ▫ joins monomers by “taking” H 2 O out one monomer donates OH– other monomer donates H+ together these form H 2 O ▫ requires energy & enzymes HO H 2 O H HO Dehydration synthesis H enzyme Condensation reaction HO H
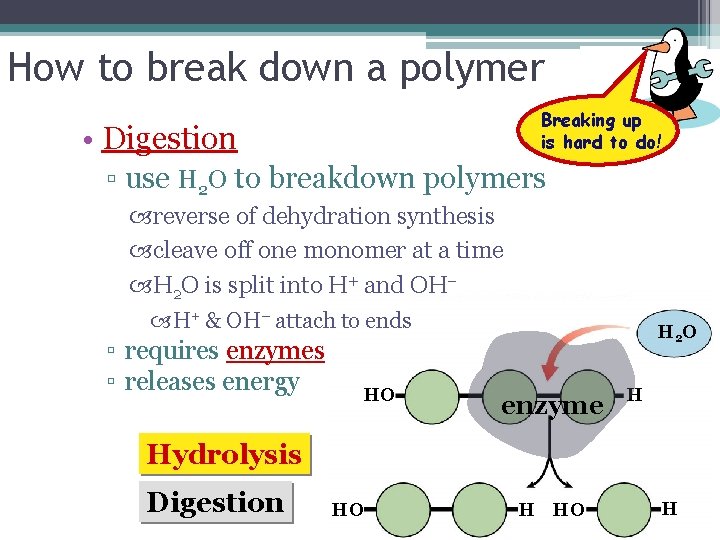
How to break down a polymer Breaking up is hard to do! • Digestion ▫ use H 2 O to breakdown polymers reverse of dehydration synthesis cleave off one monomer at a time H 2 O is split into H+ and OH– H+ & OH– attach to ends ▫ requires enzymes ▫ releases energy HO H 2 O enzyme H Hydrolysis Digestion HO H
- Slides: 24