Unit 1 BComposition of Matter Elements and compounds

Unit 1 BComposition of Matter
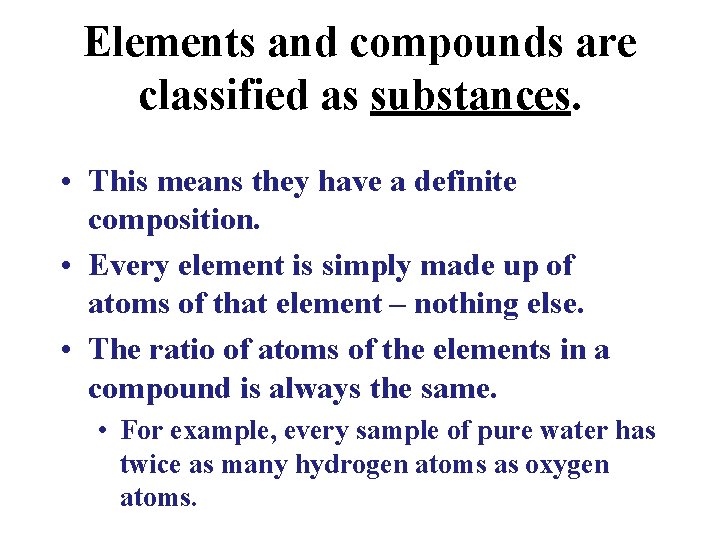
Elements and compounds are classified as substances. • This means they have a definite composition. • Every element is simply made up of atoms of that element – nothing else. • The ratio of atoms of the elements in a compound is always the same. • For example, every sample of pure water has twice as many hydrogen atoms as oxygen atoms.

Law of Definite Proportions The ratio of the masses of the constituent elements in any pure sample of that compound is always the same.
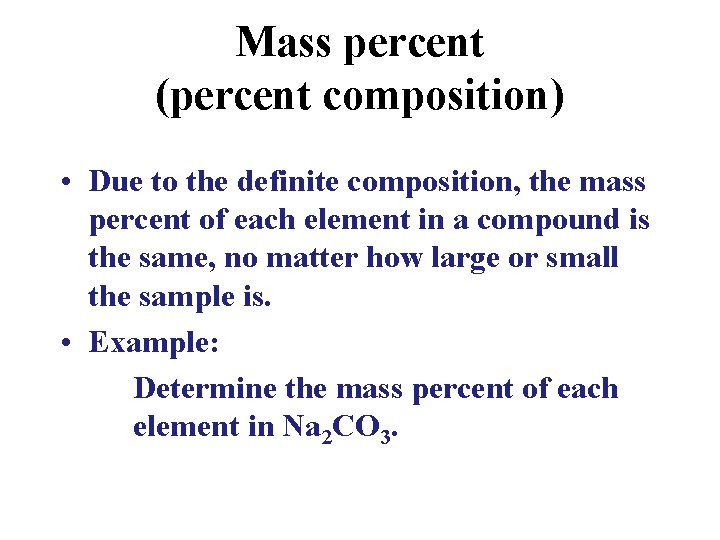
Mass percent (percent composition) • Due to the definite composition, the mass percent of each element in a compound is the same, no matter how large or small the sample is. • Example: Determine the mass percent of each element in Na 2 CO 3.
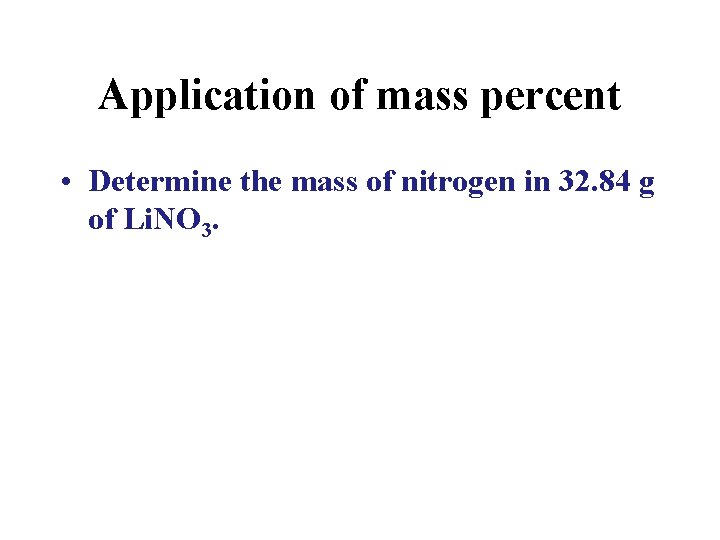
Application of mass percent • Determine the mass of nitrogen in 32. 84 g of Li. NO 3.
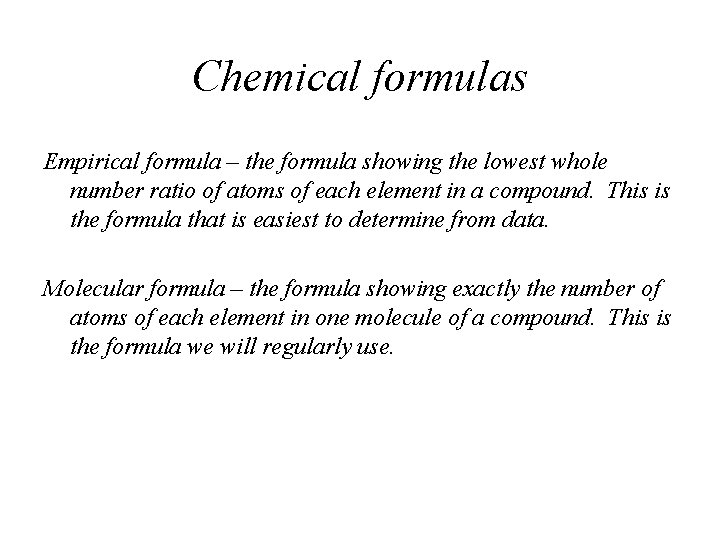
Chemical formulas Empirical formula – the formula showing the lowest whole number ratio of atoms of each element in a compound. This is the formula that is easiest to determine from data. Molecular formula – the formula showing exactly the number of atoms of each element in one molecule of a compound. This is the formula we will regularly use.
Empirical formulas In many cases, the molecular formula for a compound is the same as the empirical formula. For example – H 2 O, CH 4, SO 3, C 12 H 22 O 11 However, many molecules contain multiples of atoms that can be reduced to a smaller ratio. For example – C 6 H 12 O 6, C 3 H 6, Hg 2 Cl 2, N 2 O 4 What are the empirical formulas for these examples? Molecular formula = (empirical formula)n
Determining empirical formulas • Analysis of unknown chemicals can provide mass percents. • We can use the mass percents, or masses, to determine the ratio of moles of each element in a compound. • The whole number ratio of moles is the same as the ratio of atoms. • Empirical formulas show the ratio of atoms in a compound.
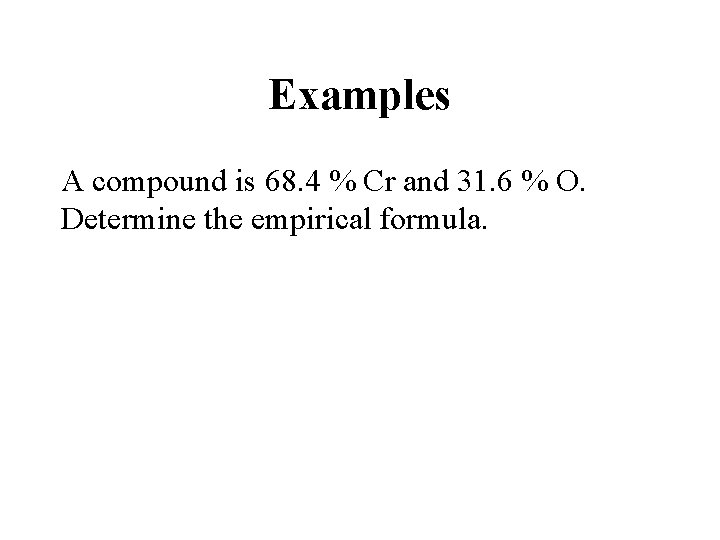
Examples A compound is 68. 4 % Cr and 31. 6 % O. Determine the empirical formula.
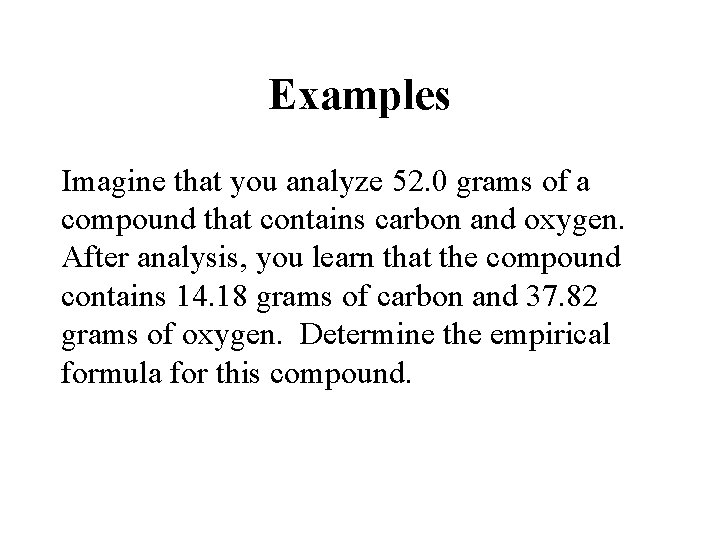
Examples Imagine that you analyze 52. 0 grams of a compound that contains carbon and oxygen. After analysis, you learn that the compound contains 14. 18 grams of carbon and 37. 82 grams of oxygen. Determine the empirical formula for this compound.
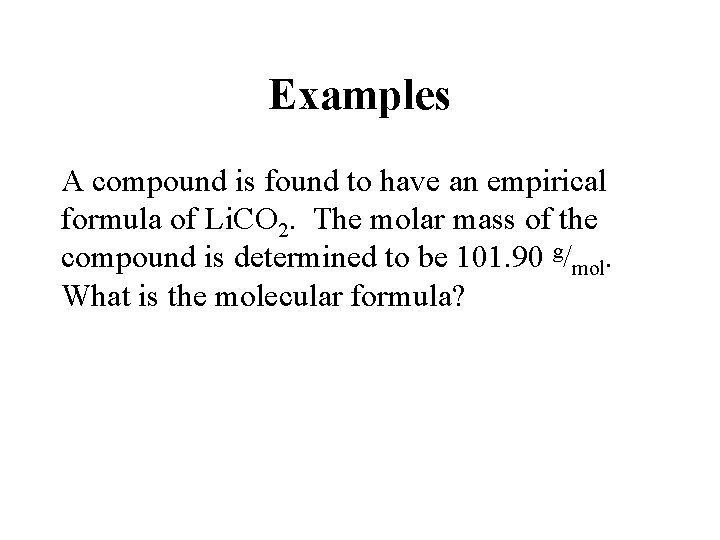
Examples A compound is found to have an empirical formula of Li. CO 2. The molar mass of the compound is determined to be 101. 90 g/mol. What is the molecular formula?
- Slides: 11