Unit 1 Atomic Structure Electron Configuration I Theories




- Slides: 27

Unit 1: Atomic Structure & Electron Configuration

I. Theories and Models Ø Scientific Model – A pattern, plan, representation or description designed to show the structure or workings of an object, system or concept.

A. Greeks • • • 400 B. C. Democritus particle theory- matter could not be divided into smaller and smaller pieces forever, eventually the smallest possible piece would be obtained and would be indivisible. called nature’s basic particle atomos-indivisible no experimental evidence to support theory

B. John Dalton • • • 1808 English school teacher Established first atomic theory: 1. 2. 3. 4. 5. • Matter is composed of atoms. Atoms of a given element are identical to each other, but different from other elements. Atoms cannot be divided nor destroyed. Atoms of different elements combine in simple whole-number ratios to form compounds. In chemical reactions, atoms are combined, separated or rearranged. Model: tiny, hard, solid sphere

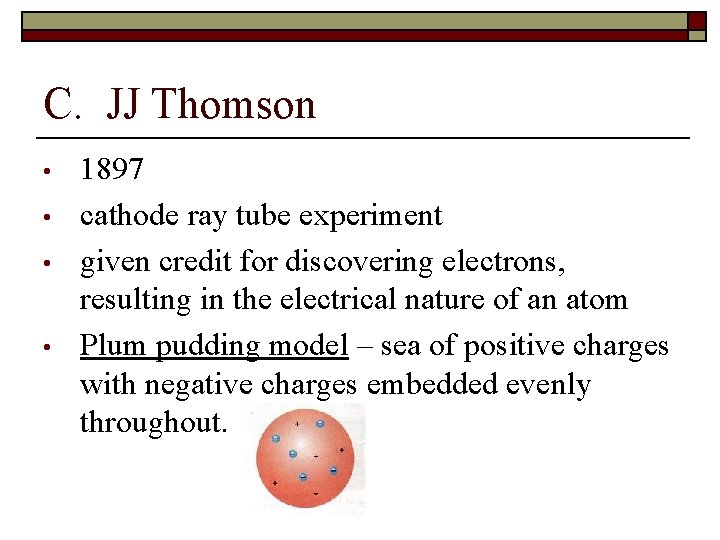
C. JJ Thomson • • 1897 cathode ray tube experiment given credit for discovering electrons, resulting in the electrical nature of an atom Plum pudding model – sea of positive charges with negative charges embedded evenly throughout.

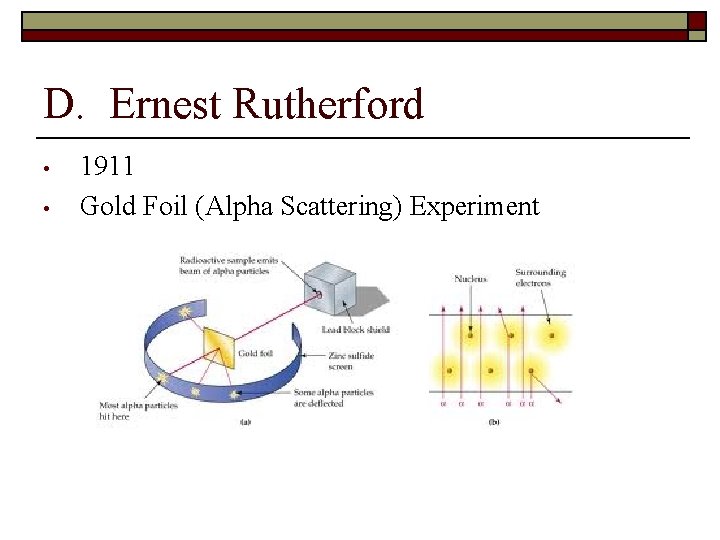
D. Ernest Rutherford • • 1911 Gold Foil (Alpha Scattering) Experiment

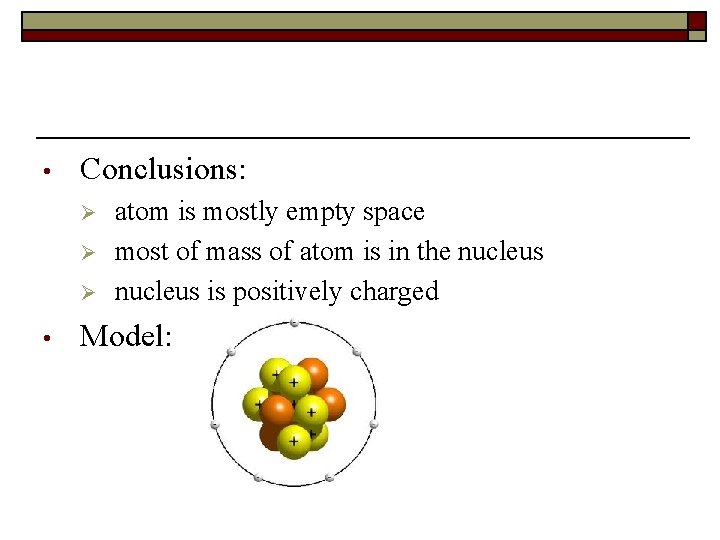
• Conclusions: Ø Ø Ø • atom is mostly empty space most of mass of atom is in the nucleus is positively charged Model:

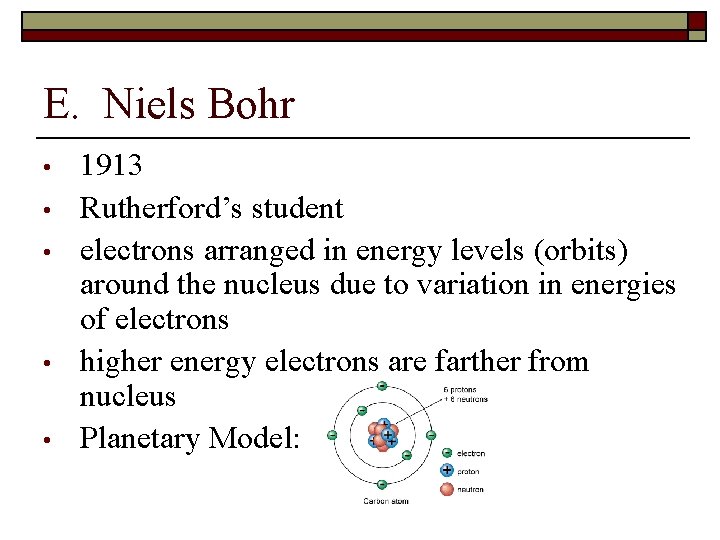
E. Niels Bohr • • • 1913 Rutherford’s student electrons arranged in energy levels (orbits) around the nucleus due to variation in energies of electrons higher energy electrons are farther from nucleus Planetary Model:

F. Quantum Model • • • 1924 -current Collaboration of many scientists Better than Bohr’s model because it describes the arrangement of e- in atoms other than H Based on the probability (95% of time) of finding and e- or an e- pair in a 3 D region around the nucleus known as an orbital Model (on board)

II. General Structure of Atom nucleus Ø Ø Ø center of atom p+ & n 0 located here positive charge most of mass of atom, tiny volume very dense e- cloud Ø Ø Ø surrounds nucleus e- located here negative charge most of volume of atom, negligible mass low density

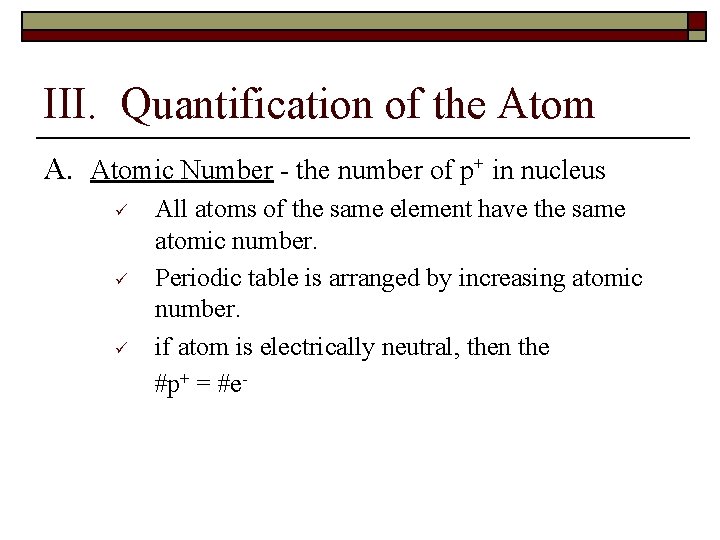
III. Quantification of the Atom A. Atomic Number - the number of p+ in nucleus ü ü ü All atoms of the same element have the same atomic number. Periodic table is arranged by increasing atomic number. if atom is electrically neutral, then the #p+ = #e-

B. Mass Number - the total number of p+ & n 0 in nucleus of an atom. ü ü Round the atomic weight to a whole number n 0 = mass number - atomic number

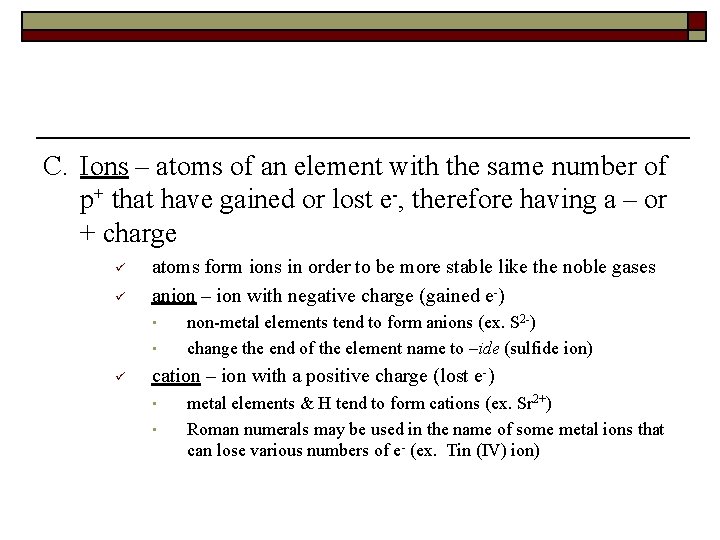
C. Ions – atoms of an element with the same number of p+ that have gained or lost e-, therefore having a – or + charge ü ü atoms form ions in order to be more stable like the noble gases anion – ion with negative charge (gained e-) • • ü non-metal elements tend to form anions (ex. S 2 -) change the end of the element name to –ide (sulfide ion) cation – ion with a positive charge (lost e-) • • metal elements & H tend to form cations (ex. Sr 2+) Roman numerals may be used in the name of some metal ions that can lose various numbers of e- (ex. Tin (IV) ion)

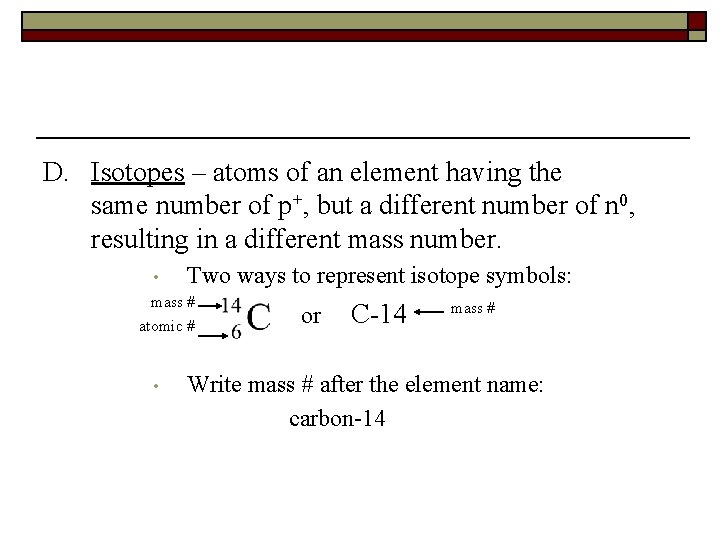
D. Isotopes – atoms of an element having the same number of p+, but a different number of n 0, resulting in a different mass number. • Two ways to represent isotope symbols: mass # atomic # • or C-14 mass # Write mass # after the element name: carbon-14

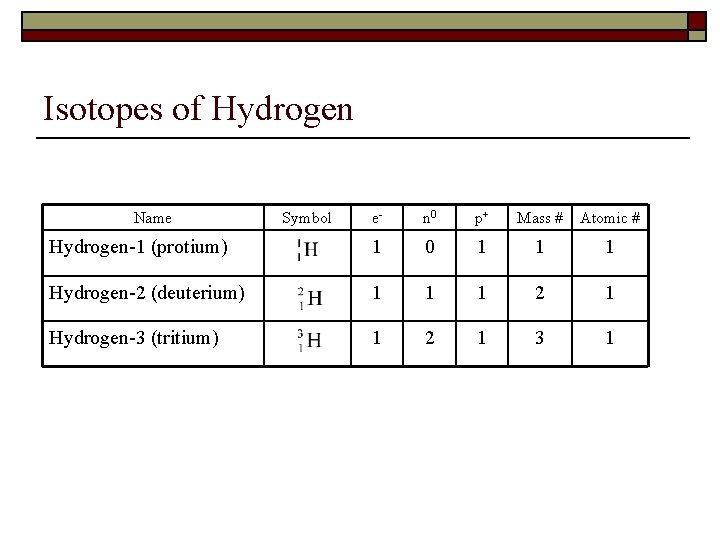
Isotopes of Hydrogen Name Symbol e- n 0 p+ Mass # Atomic # Hydrogen-1 (protium) 1 0 1 1 1 Hydrogen-2 (deuterium) 1 1 1 2 1 Hydrogen-3 (tritium) 1 2 1 3 1

E. Average Atomic Mass – weighted average of all natural isotopes of an element expressed in amu* (atomic mass units). Ø Ø based on % abundance of isotopes steps for calculating: 1. 2. 3. 4. change % to decimal multiply decimal and mass number add all results place amu unit with answer *amu=1/12 mass of C-12 isotope

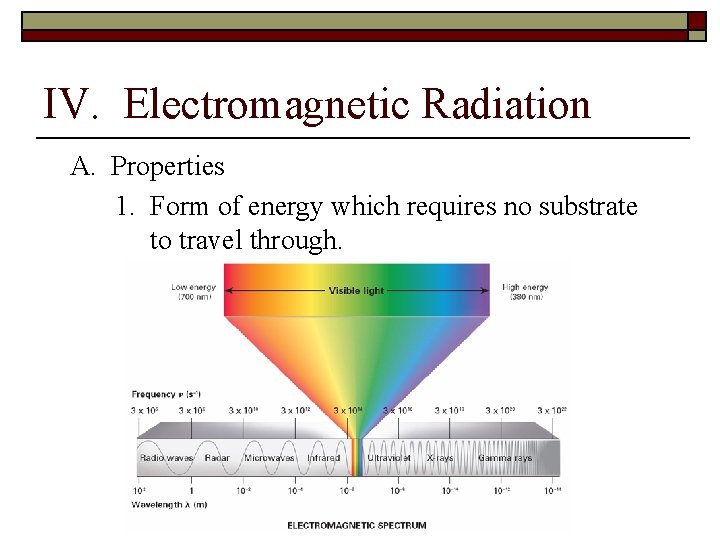
IV. Electromagnetic Radiation A. Properties 1. Form of energy which requires no substrate to travel through.

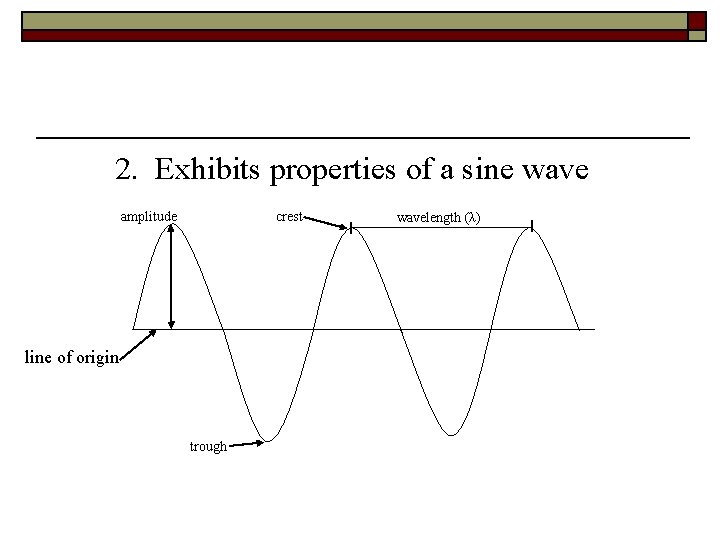
2. Exhibits properties of a sine wave crest amplitude line of origin trough wavelength (λ)

a. wavelength = distance between consecutive crests (Greek letter lambda = λ) b. frequency = # wave cycles passing a given point over time (seconds); (Greek letter nu = ν ) *measured in Hertz (Hz)= 1/s, s-1, or per second c. all types of ER travel in a vacuum at the speed of light (c) = 3. 00 x 108 m/s

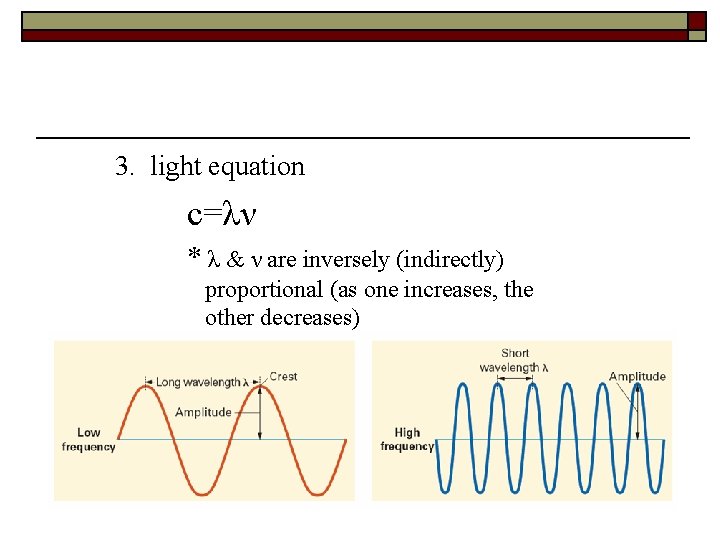
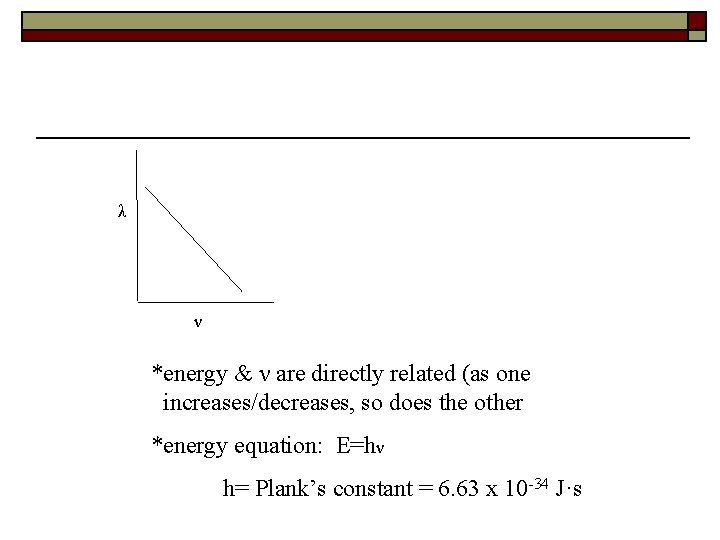
3. light equation c=λν * λ & ν are inversely (indirectly) proportional (as one increases, the other decreases)

λ ν *energy & ν are directly related (as one increases/decreases, so does the other *energy equation: E=hν h= Plank’s constant = 6. 63 x 10 -34 J·s

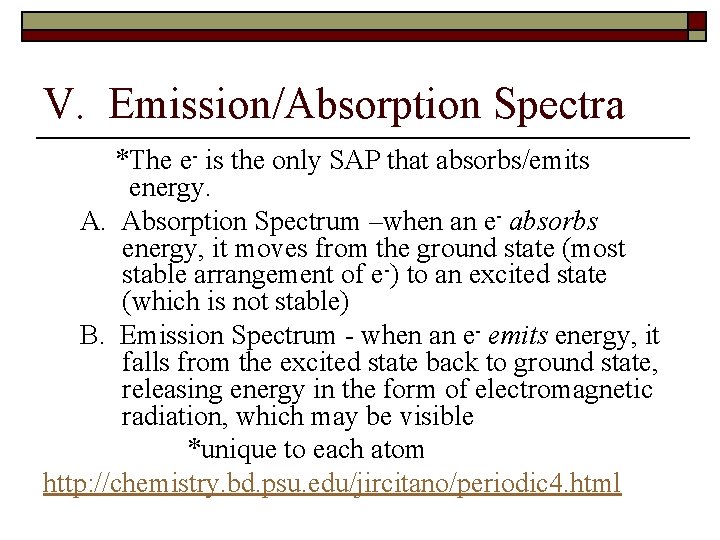
V. Emission/Absorption Spectra *The e- is the only SAP that absorbs/emits energy. A. Absorption Spectrum –when an e- absorbs energy, it moves from the ground state (most stable arrangement of e-) to an excited state (which is not stable) B. Emission Spectrum - when an e- emits energy, it falls from the excited state back to ground state, releasing energy in the form of electromagnetic radiation, which may be visible *unique to each atom http: //chemistry. bd. psu. edu/jircitano/periodic 4. html

VI. Electron Configuration A. Describes the arrangement of e- in an atom 1. each main energy level is divided into sublevels 2. each sublevel is made up of orbitals, each of which can hold up to 2 e*chart

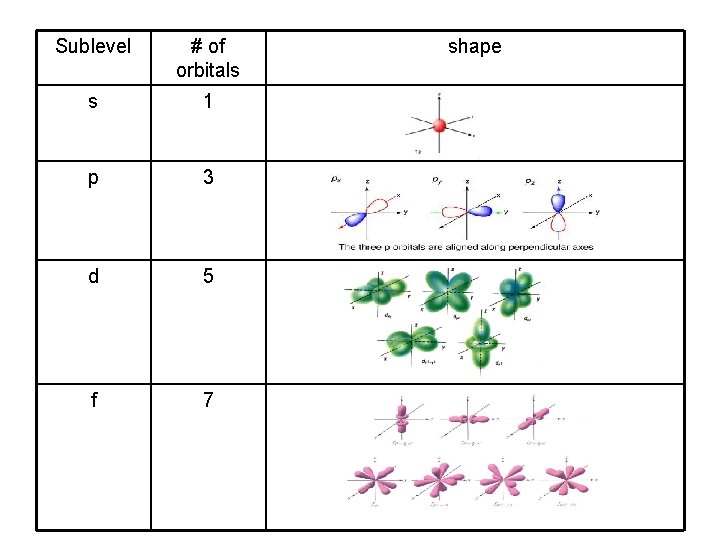
Sublevel # of orbitals s 1 p 3 d 5 f 7 shape

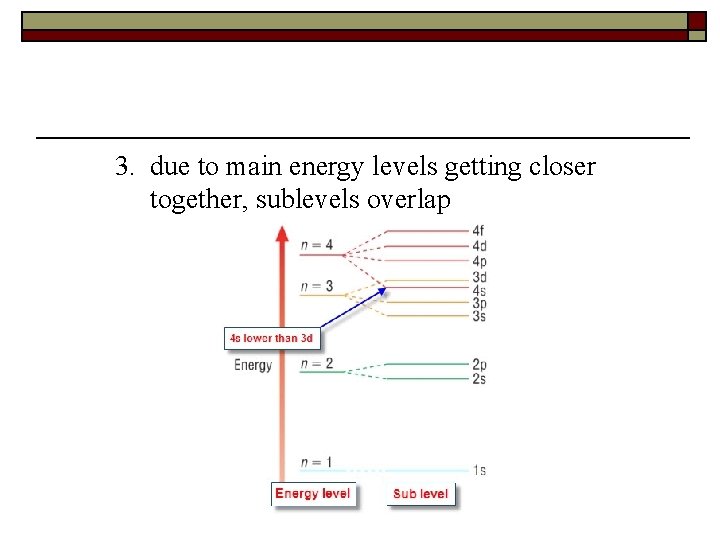
3. due to main energy levels getting closer together, sublevels overlap

4. Aufbau principle – states that e- fill orbitals of lower energy sublevels first 5. Abbreviated Configurations – use the preceding noble gas symbol (in brackets) to represent the filled inner core of e-. Then write the remaining configuration for the atom.

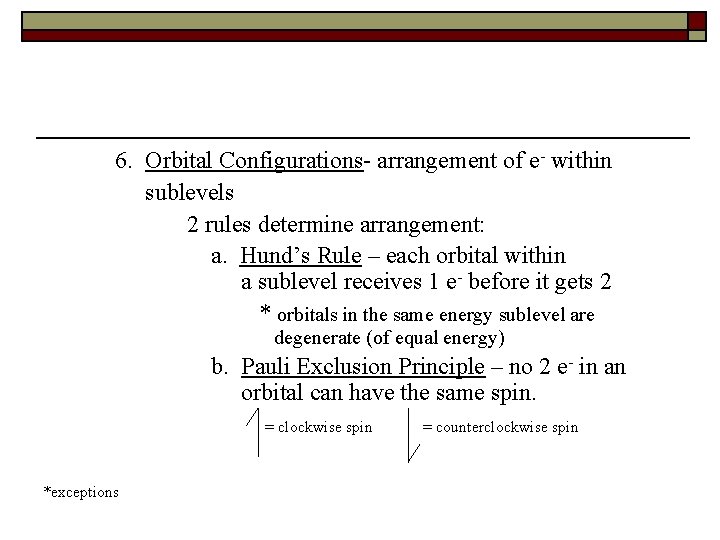
6. Orbital Configurations- arrangement of e- within sublevels 2 rules determine arrangement: a. Hund’s Rule – each orbital within a sublevel receives 1 e- before it gets 2 * orbitals in the same energy sublevel are degenerate (of equal energy) b. Pauli Exclusion Principle – no 2 e- in an orbital can have the same spin. = clockwise spin *exceptions = counterclockwise spin