UNIT 1 ACIDS ALKALIS CHEMICAL REACTIONS Lesson 5







- Slides: 9

UNIT 1 ACIDS, ALKALIS & CHEMICAL REACTIONS Lesson 5 – Uses of Neutralisation

Aims of the Lesson • I know that there are practical uses for the process of neutralisation. • I can give examples of these uses and explain why they are important.

Discuss • In groups of 2 -4 people spend 10 minutes discussing the following: 1. What practical application might there be for the neutralisation process in everyday life? 2. How are these carried out? 3. Do you think these processes are important? Why? 4. If you are stuck use the Intermediate 1 chemistry textbook page ____ Think of as many examples as you can. Each group will report back on one idea to the class.

Digestion • Our digestive system breaks down food by action of bilic acid produced in the stomach. • Sometimes ‘rich’ food can cause the stomach to become too acidic (p. H 1 to 6) which causes ‘indigestion’. • An indigestion tablet is often eaten (Setlers, Rennie, Remegel etc) which contains alkali (p. H 8 to 14) to reduce the over-acidity of the stomach. • Neutralisation of the stomach would occur when the stomach reaches p. H 7.

Soil Treatment • Soil can be mixed with water and tested. Often soil is found to be acidic (about p. H 4). This occurs when acid rain falls on the ground. • To reduce the acidity of the soil an alkali has to be added to help plant growth as acid kills the roots. • Soda (lime) can be mixed in with the soil and water. Farmers would do this on a large scale in the fields. • As soda (lime) is a natural alkali of p. H 11 the acidity of the soil is reduced and is neutralised when it ends up with a p. H 7 value.

River/Lake Treatment • Water in rivers and lakes can also be tested. It too is often found to be acidic (about p. H 4). This occurs when acid rain falls in to the water supply. • To reduce the acidity of the water an alkali has to be added to help maintain healthy fish stocks. • Soda (lime) can be mixed in with the rivers and lakes. • As soda (lime) is a natural alkali of p. H 11 the acidity of the soil is reduced and is neutralised when it ends up with a p. H 7 value.

EXPERIMENT 1. Collect the following equipment: small beaker, bottle of hydrochloric acid, universal indicator and indigestion tablet. 2. Pour approx 50 cm 3 of acid into the beaker. 3. Add universal indicator. 4. Now add the indigestion tablet. Watch what happens. What is happening?

Summary • Complete the following in your jotter: When the universal indicator was added to the beaker it turned ______. This happened because _____ acid was present. When the indigestion tablet was added the colour changed to ______. This indicated that the p. H must be____ and that a _____ reaction was occurring.

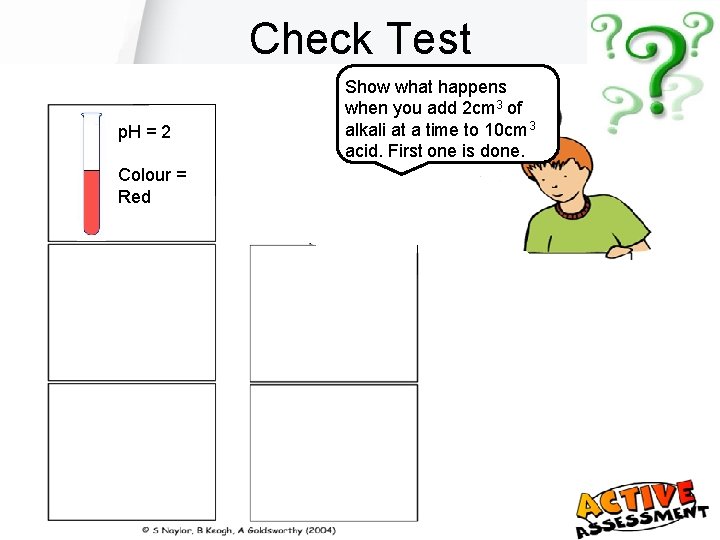
Check Test p. H = 2 Colour = Red Show what happens when you add 2 cm 3 of alkali at a time to 10 cm 3 acid. First one is done.
 Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Hso4na
Hso4na Everyday alkalis
Everyday alkalis Types of reactions
Types of reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations We add water to acid
We add water to acid Dangerous alkalis
Dangerous alkalis
















