UNIT 1 6 BONDING 1 6 Bonding Students

UNIT 1. 6 BONDING

1. 6 Bonding Students will be assessed on their ability to: 1 Ionic bonding a. recall and interpret evidence for the existence of ions, limited to physical properties of ionic compounds, electron density maps and the migration of ions, eg electrolysis of aqueous copper chromate(VI) describe the formation of ions in terms of electron loss or gain draw electron configuration diagrams of cations and anions using dots or crosses to represent electrons describe ionic crystals as giant lattices of ions describe ionic bonding as the result of strong net electrostatic attraction between ions recall trends in ionic radii down a group and for a set of isoelectronic ions, eg N 3 - to Al 3+ recall the stages involved in the formation of a solid ionic crystal from its elements and that this leads to a measure value for the lattice energy (students will not be expected to draw the full Born-Haber cycles) test the ionic model for ionic bonding of a particular compound by comparison of lattice energies obtained from the experimental values used in Born-Haber cycles, with provided values calculated from electrostatic theory explain the meaning of the term polarization as applied to ions demonstrate an understanding that the polarizing power of a cation depends on its radius and charge, and the polarizability of an anion depends on its size demonstrate an understanding that polarization of anions by cations leads to some covalency in an ionic bond, based on evidence from the Born-Haber cycle use values calculated for standard heats of formation based on Born-Haber cycles to explain why particular ionic compounds exist, eg the relative stability of Mg. Cl 2 over Mg. Cl or Mg. Cl 3 and Na. Cl over Na. Cl 2. b. c. d. e. f. g. h. i. j. k. l.

2 Covalent bonding a. demonstrate an understanding that covalent bonding is strong and arises from the electrostatic attraction between the nucleus and the electrons which are between the nuclei, based on the evidence: i. the physical properties of giant atomic structures ii. electron density maps for simple molecules b. draw electron configuration diagrams for simple covalently bonded molecules, including those with multiple bonds and dative covalent bonds, using dots or crosses to represent electrons. 3 Metallic bonding a. demonstrate an understanding that metals consist of giant lattices of metal ions in a sea of delocalised electrons b. describe metallic bonding as the strong attraction between metal ions and the sea of delocalised electrons c. use the models in 1. 6. 3 a and 1. 6. 3 b to interpret simple properties of metals, eg conductivity and melting temperatures.

Recap of GCSE Electronic Configurations Hydrogen Helium Lithium Beryllium Boron H He Li Be B H: 1 He: 2 Li: 2. 1 Be: 2. 2 B: 2. 3 Nitrogen N N: 2. 5 Oxygen O O: 2. 6 Carbon C C: 2. 4 Fluorine Neon Sodium F Ne Na F: 2. 7 Ne: 2. 8 Na: 2. 8. 1

Magnesium Mg Aluminium Al Silicon Phosphorus Si P P: 2. 8. 5 Mg: 2. 8. 2 Al: 2. 8. 3 Si: 2. 8. 4 Sulphur Chlorine Argon S Cl Ar S: 2. 8. 6 Cl: 2. 8. 7 Ar: 2. 8. 8

Potassium K K: 2. 8. 8. 1 Calcium Ca Ca: 2. 8. 8. 2

IONIC BONDING Bonding between metals and non metals forming ions Q: Draw the ionic bonding in lithium fluoride. State the ions produced and give the molecular formula of lithium fluoride 1. Draw the electronic configuration for lithium 2. Draw the electronic configuration for fluorine Li Formula of lithium fluoride is Li. F F

Q: Draw the ionic bonding in magnesium oxide. State the ions produced and give the molecular formula of magnesium oxide. 2 2 O Mg Formula for magnesium oxide is Mg. O Q: Draw the ionic bonding in calcium chloride. State the ions produced and give the molecular formula of calcium chloride. 2 Ca Cl Cl Formula for calcium chloride is Ca. Cl 2 Q: Draw the ionic bonding in aluminium oxide. State the ions produced and give the molecular formula of aluminium oxide.
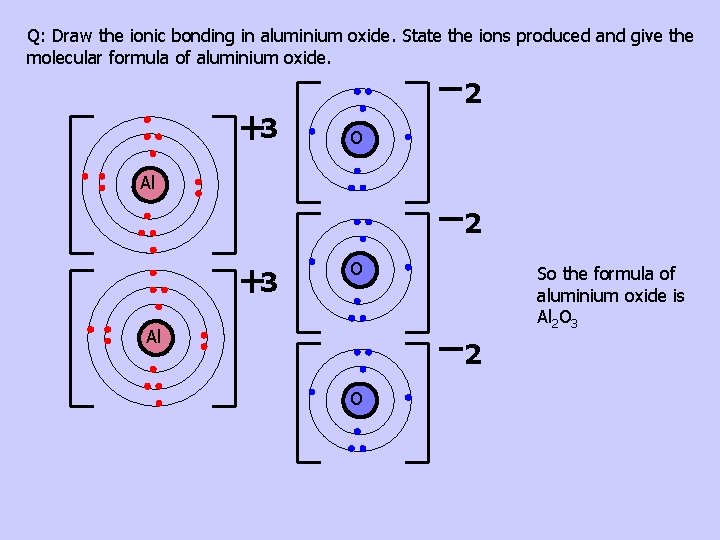
Q: Draw the ionic bonding in aluminium oxide. State the ions produced and give the molecular formula of aluminium oxide. 2 3 O Al So the formula of aluminium oxide is Al 2 O 3 2 O

What are Ions? Ions are charged atoms or molecules. There are 2 types of ion: Cation Anion Formed by losing electron(s) Formed by gaining electron(s) Negatively charged Positively charged Metal ions Non-metal ions

How Big are Ions? Ionic Radii measured in nm Cations Na Na+ Anions F 0. 072 0. 192 0. 095 Mg Mg 2+ O 0. 160 0. 065 0. 066 Cations are smaller than their atoms F 0. 136 O 20. 140 Anions are larger than their atoms

What is an Ionic Bond? “The net electrostatic attraction between oppositely charged ions” Eg Na. Cl: + - + GIANT IONIC LATTICE Giant ionic lattices are made of millions of oppositely charged ions and their molecular formula is the simplest ratio of the ions. (I. e. the empirical formula!)

How to Draw a Giant Covalent Lattice E. g:

Strength of Ionic Bonds The stronger the attraction between the ions, the stronger the ionic bond What 2 factors affect the force of attraction? Length of the bond AKA Size of the ion Smaller ions = Stronger bond Charge on the ions Higher charge = Stronger bond These two factors together we call CHARGE DENSITY The higher the charge density = Stronger bond

Ionic Radii Down a Group Li+ 0. 068 Na+ Increases down the group 0. 095 WHY? K+ 0. 133 Rb+ 0. 148 Cs+ 0. 169 More shells of electrons! The 3 rd shell is further from the nucleus than the 2 nd etc.

Ionic Radii of Isoelectronic Ions Iso = The same Electronic = electrons All of the following ions have the same electronic configuration: 1 s 2 2 p 6 N 3 - 0. 171 O 20. 140 F- Na+ 0. 136 0. 095 Mg 2+ Al 3+ 0. 065 0. 050 Increases with increasing negative charge

Melting Points of Some Ionic Structures Sodium chloride Potassium chloride Calcium oxide Magnesium oxide Ions Na+ Cl. K+ Cl. Ca 2+ O 2 Mg 2+ O 2 - Ionic Radius (nm) Melting Point 0 C 0. 095 801 0. 181 0. 133 0. 181 0. 099 0. 140 0. 065 0. 140 776 782 2614 2800

Properties of Ionic Compounds High melting/boiling points-millions of strong bonds need to be broken Don’t conduct electricity as electrons are not free to move through the solid Conduct electricity when molten and in aqueous solution as ions are free to move Dissolve* in water and other molten ionic compounds (hydration bonds) Brittle due to repulsion of ions when hit HIT IONS MOVE ALONG REPULSION

Polarisation of Ionic Bonds Ionic bonding is not quite so straightforward The ions can affect those that are next to them Positive ions attract the electrons in the negative ion POLARISING it The ions can affect those that are next to them + - Perfect ionic Increasingly polarised anion + + - -

Polarising Polarisable Small Large Positive Negative Out of the following pairs of ions which would be MOST polarisable or polarising 1 K+ Na+ Most polarising 2 O 2 - S 2 - Most polarisable 3 Be 2+ Li+ Most polarising 4 I- Most polarisable 5 Al 3+ Ca 2+ Most polarising 6 7 S 2 - Br. Rb+ B 3+ Most polarisable Cl- Most polarising

Lattice Enthalpy Used to quantify the strength of an ionic bond. It gives a measure of the force of attraction between the positive and negative ions in the lattice Definition: The Standard Lattice Energy ∆Hөlat is the Enthalpy change when 1 mole of solid ionic lattice is formed from completely separated ions in the gaseous state under standard conditions A+(g) + B-(g) AB(s) E. g. Na+(g) + Cl-(g) g Na. Cl(s)

More Definitions you Should Know from Energetics Atomisation Enthalpy ∆Hөat is the Enthalpy change when 1 mole of gaseous atoms is formed from an element in its standard state A(s) A(g) E. g. Na(s)g Na(g) E. g. ½Cl 2(g) g Cl(g) First Ionisation Enthalpy ∆HөIE 1 is the Energy required to remove one mole of electrons from one mole of gaseous atoms to form one mole of unipositive gaseous ions A(g) A+(g) + e- E. g. Na(g) g Na+(g) + e- Second Ionisation Enthalpy ∆HөIE 2 is the Energy required to remove one mole of electrons from one mole of gaseous unipositive ions form one mole of dipositive gaseous ions A+(g) A 2+(g) + e- E. g. Na+(g) g Na 2+(g) + e-

More Definitions you Should Know from Energetics First Electron Affinity ∆HөEA 1 is the Energy change when 1 mole of electrons is added to one mole of gaseous atoms forming one mole of uninegative gaseous ions A(g) + e- A-(g) E. g. Cl(g) + e- g Cl-(g) Second Electron Affinity ∆HөIE 2 is the Energy change when 1 mole of electrons is added to one mole of gaseous uninegative ions forming one mole of dinegative ions ALWAYS ENDOTHERMIC! A-(g) + e- A 2 -(g) E. g. Cl-(g) + e- g Cl 2 -(g) Enthalpy of Formation ∆Hөf is the Energy change when one mole of a compound is formed from its constituent elements in their standard states A(s) + B 2(g) AB 2(s) E. g. Na(s) + ½Cl 2(g) g 2 Na. Cl(s)

How can we Calculate Lattice Enthalpies? (A special type of enthalpy level diagram) Reactants Exothermic Recation Energy/Enthalpy Activation energy Born-Haber Cycles Products

How can we Calculate Lattice Enthalpies? Born-Haber Cycle of Sodium Chloride Na(s) + ½Cl 2(g) Exothermic Recation Energy/Enthalpy Activation energy See born haber cycle definitions Na. Cl(s)

How can we Calculate Lattice Enthalpies? Born-Haber Cycle of Sodium Chloride Atomisation First Electron Enthalpy Lattice Ionisation enthalpy of Formation of. Affinity Sodium Chlorine Enthalpy ofof. Chlorine of Sodium chloride Energy/Enthalpy Na+(g) + Cl(g) + e. Na(g) + Cl(g) Na(g) + ½Cl 2(g) Na(s) + ½Cl 2(g) DHIE 1(Na) DHat(Cl) Exothermic Endothermic DHEA 1(Cl) Na+(g) + Cl- (g) DHat(Na) DHlat(Na. Cl) DHf(Na. Cl) Na. Cl(s) You don’t need to remember this, just be aware of the different endo/exothermic stages of the cycle

Why does Mg. Cl 2 form rather than Mg. Cl or Mg. Cl 3, and Na. Cl over Na. Cl 2? Mg 2+(g) + 2 Cl(g) + 2 e- Energy/Enthalpy Mg+ (g) DH - IE 2(Mg) 2 DHEA 1(Cl) + 2 Cl(g) + e Mg(g) + 2 Cl(g) Mg(g) + Cl 2(g) Mg(s) + Cl 2(g) DHIE 1(Mg) Mg 2+(g) + 2 Cl- (g) 2 DHat(Cl) DHat(Mg) DHlat(Mg. Cl ) 2 DHf(Mg. Cl ) 2 Mg. Cl 2(s)

See born haber cycles in notebook

Difference Between Theoretical and Calculated Lattice Enthalipes Theoretical Calculated Based on pure 100% perfect ionic bonding Based on actual experimental results The larger the difference between these two lattice enthalpies , the more polarisation that must be in the lattice, which leads to “A greater degree of covalency” The calculated lattice enthalpy for magnesium chloride is -2526 k. Jmol -1, whereas theoretical lattice enthalpy is -2326 k. Jmol-1. Explain this difference. (3) The theoretical value assumes perfect ionic bonding The small highly charged magnesium ion polarises the chloride ion Causing a degree of covalent bonding, which causes the difference

Electrolysis of Ionic Compounds

Hydrogen Gas (H 2) Chlorine Gas (Cl 2) THE IONS Sodium (Na+) Chloride (Cl-) Hydroxide (OH-) Hydrogen (H+) Water naturally Splits into ions: H 2 O g H+ + OH- Sodium Hydroxid e Solution (Na. OH) Cathode Anode

Hydrogen Gas (H 2) Chlorine Gas (Cl 2) THE IONS Sodium (Na+) Chloride (Cl-) Hydroxide (OH-) Hydrogen (H+) Water naturally Splits into ions: H 2 O g H+ + OH- Sodium Hydroxide Solution (Na. OH) Cathode Anode

Hydrogen Gas (H 2) Chlorine Gas (Cl 2) THE IONS Sodium (Na+) Chloride (Cl-) Hydroxide (OH-) Hydrogen (H+) Water naturally Splits into ions: H 2 O g H+ + OH- Sodium Hydroxide Solution (Na. OH) Cathode Anode

Electrolysis of Copper chromate(VI) (Cu. Cr. O 4)

The electrolysis of copper(II) chromate(VI) Copper chromate is a dark green solid, which can be bought or made by mixing copper sulfate with potassium chromate. Passing an electric current through a specially prepared copper chromate solution results in the migration of two coloured ions. The green solution produces two bands of colour: blue copper cations and yellow chromate anions. Kit U-tube; two graphite rods with slit corks to fit the U-tube 100 V dc supply; electric cables Retort stand, boss and clamp Pipette Solid Cu. Cr. O 4 Ammonia solution, 2 mol dm-3 Urea

Procedure Make the copper(II) chromate(VI) solution by dissolving solid Cu. Cr. O 4 in the minimum amount of ammonia solution and then saturating with urea to increase its density. Alternatively, you can mix 100 cm 3 of molar solutions of copper sulfate and potassium chromate. An orange-brown precipitate will form by double decomposition. Filter the solution through a Buchner flask and scrape the solid into a beaker containing 200 cm 3 ammonia solution (2 mol dm-3). Stir the solution using a magnetic stirrer until the solid is completely dissolved. Add urea until the solution is saturated. Clamp the U-tube to the retort stand half fill with the ammonia solution. Using a pipette carefully add the copper chromate down the side of the U-tube beneath the ammonia. Place the graphite rods in the U-tube so that they dip into the ammonia solution and connect to the dc supply. Immediately, bubbles of gas are evolved at both electrodes (H 2 at the cathode and O 2 at the anode) caused by the electrolysis of water in the solution. After a few minutes a blue band forms near the cathode and a yellow band forms near the anode.

Safety Copper and chromate salts are toxic and may be fatal if swallowed. Potassium chromate is a potential carcinogen. Contact with the eyes can cause long-term damage. Ammonia solution is corrosive and skin contact may cause burns. Concentrated solutions release dangerous amounts of ammonia vapour into the air, a hazard if inhaled. The copper chromate solution in the U-tube should be disposed of as toxic solid waste. Special tips The trick is to ensure the copper chromate is pipetted to the bottom of the U-tube without any mixing taking place so that the ammonia remains clear and colourless.

COVALENT BONDING Between non-metal atoms ONLY! E. g. Hydrogen gas (H 2) H H H Draw the bonding in the following compounds: Oxygen (O 2) Nitrogen (N 2) Water (H 2 O) Ammonia (NH 3) H

METALLIC BONDING

Ca S Mg Ar K P Na Cl Al Si Ne F O

B C N He H Be Li
- Slides: 41