UNIT 1 3 1 1 Biological molecules Introduction
UNIT 1 3. 1. 1 Biological molecules Introduction to Biological Molecules

Lesson 1: Why do we need to know about biochemistry? • STARTER: There are 16 elements essential for life. Write down what you think they might be? • The four most common elements in living organisms are, in order, hydrogen, carbon, oxygen and nitrogen. (99% of the mass in all living organisms). • What is special about these elements?
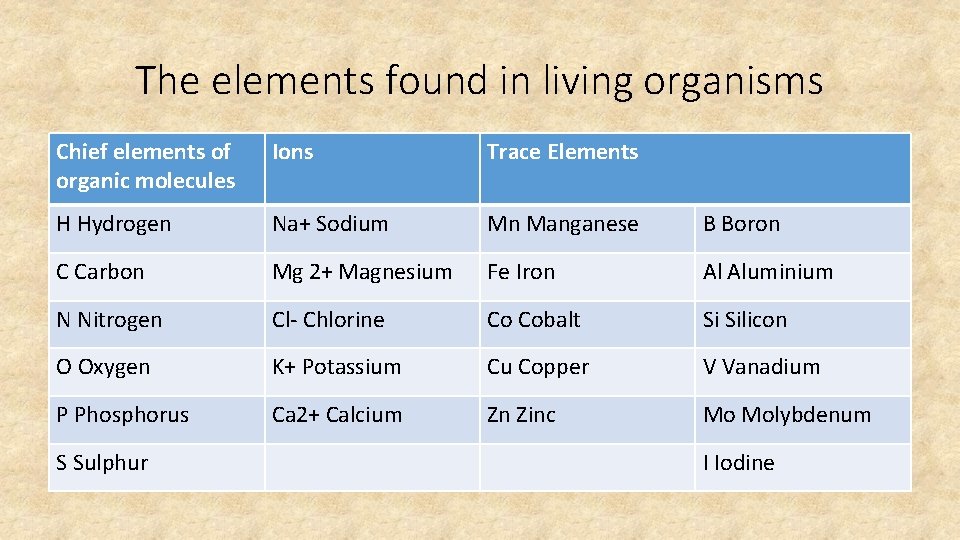
The elements found in living organisms Chief elements of organic molecules Ions Trace Elements H Hydrogen Na+ Sodium Mn Manganese B Boron C Carbon Mg 2+ Magnesium Fe Iron Al Aluminium N Nitrogen Cl- Chlorine Co Cobalt Si Silicon O Oxygen K+ Potassium Cu Copper V Vanadium P Phosphorus Ca 2+ Calcium Zn Zinc Mo Molybdenum S Sulphur I Iodine

MAIN: Revision of basic chemistry Write down definitions for the following key terms; molecule, atom, covalent bonding, ionic bonding, monomer and polymer. Support – discuss with the person next to you or your table Watch the video to recap. (https: //www. youtube. com/watch? v=R 0 g-H 1 dcfn. Y&safe=active).
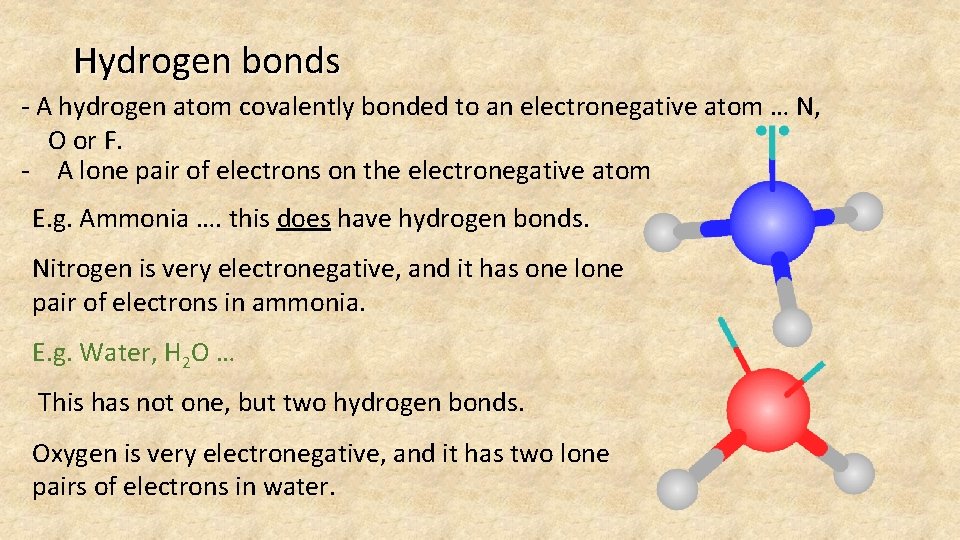
Hydrogen bonds - A hydrogen atom covalently bonded to an electronegative atom … N, O or F. - A lone pair of electrons on the electronegative atom E. g. Ammonia …. this does have hydrogen bonds. Nitrogen is very electronegative, and it has one lone pair of electrons in ammonia. E. g. Water, H 2 O … This has not one, but two hydrogen bonds. Oxygen is very electronegative, and it has two lone pairs of electrons in water.
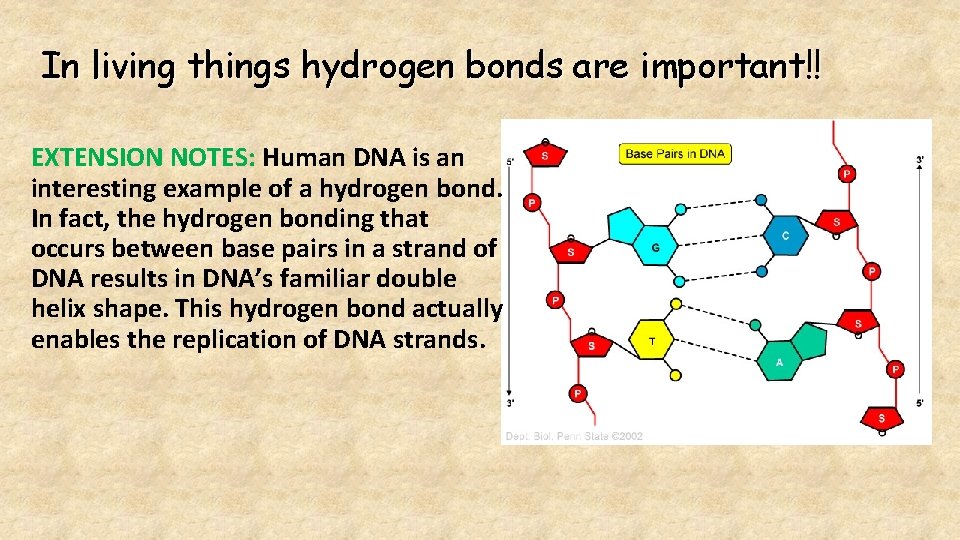
In living things hydrogen bonds are important!! EXTENSION NOTES: Human DNA is an interesting example of a hydrogen bond. In fact, the hydrogen bonding that occurs between base pairs in a strand of DNA results in DNA’s familiar double helix shape. This hydrogen bond actually enables the replication of DNA strands.
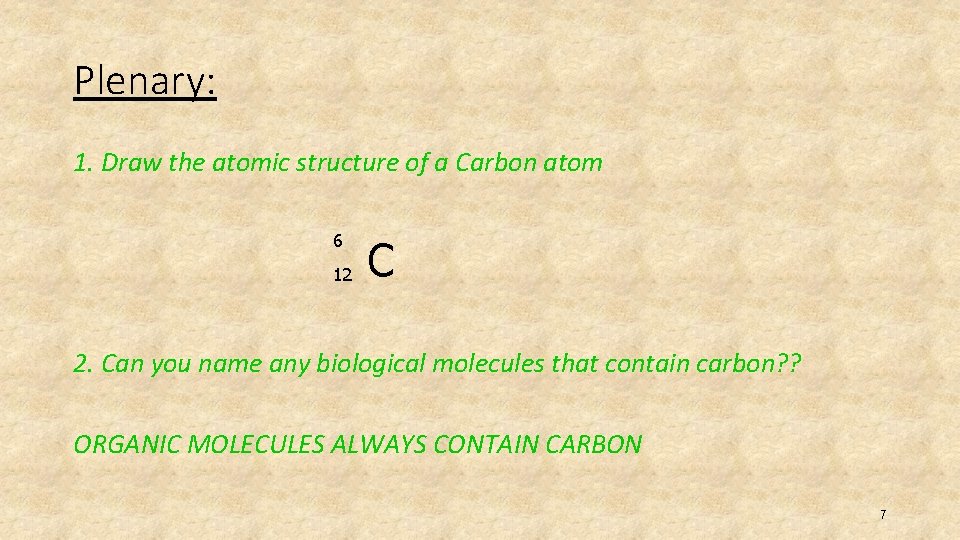
Plenary: 1. Draw the atomic structure of a Carbon atom 6 12 C 2. Can you name any biological molecules that contain carbon? ? ORGANIC MOLECULES ALWAYS CONTAIN CARBON 7
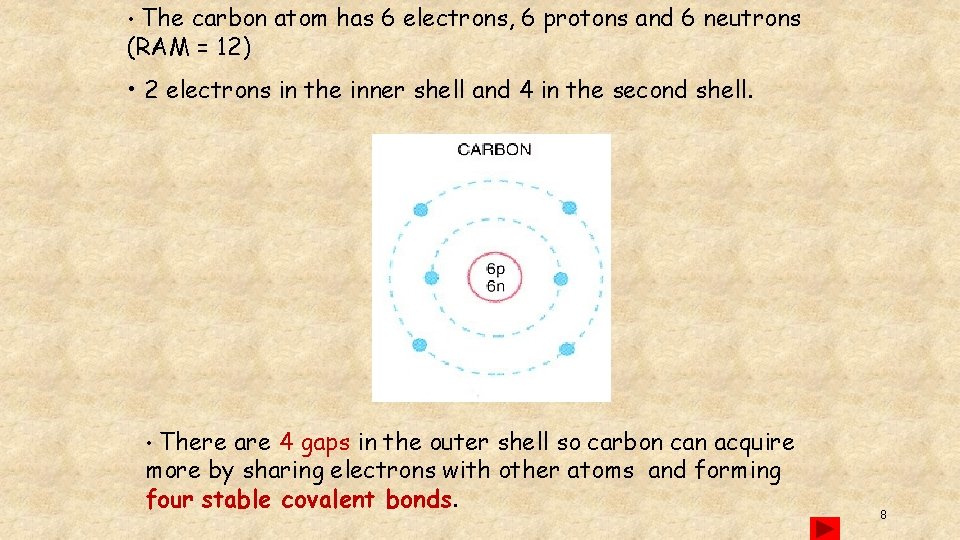
• The carbon atom has 6 electrons, 6 protons and 6 neutrons (RAM = 12) • 2 electrons in the inner shell and 4 in the second shell. • There are 4 gaps in the outer shell so carbon can acquire more by sharing electrons with other atoms and forming four stable covalent bonds. 8

Learning review: Do you …. . • understand that the variety of life is extensive but the biochemical basis of life is similar for all living things. • know that organic molecules always contain carbon. • know that carbon forms covalent bonds with other atoms Are you …. . • able to explain hydrogen bonding.
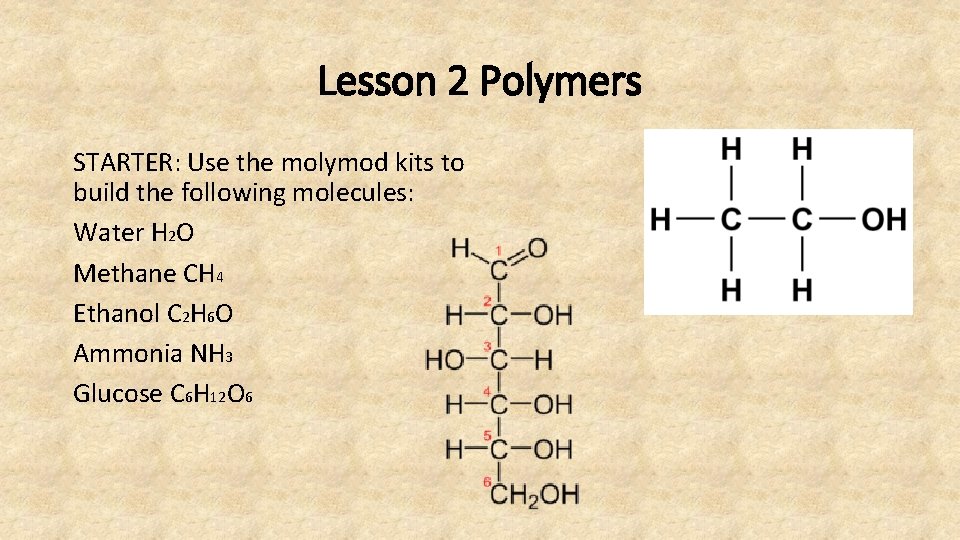
Lesson 2 Polymers STARTER: Use the molymod kits to build the following molecules: Water H 2 O Methane CH 4 Ethanol C 2 H 6 O Ammonia NH 3 Glucose C 6 H 12 O 6

Why is carbon so important? • It is a relatively small atom with little mass • It has the ability to form four strong, stable covalent bonds • It has the ability to form carbon-carbon bonds, thus building up large carbon skeletons with ring and/or chain structures. • It has the ability to form multiple covalent bonds with other carbon atoms, oxygen and nitrogen. • This allows a huge number of different types and sizes of molecule, all based on carbon. 11
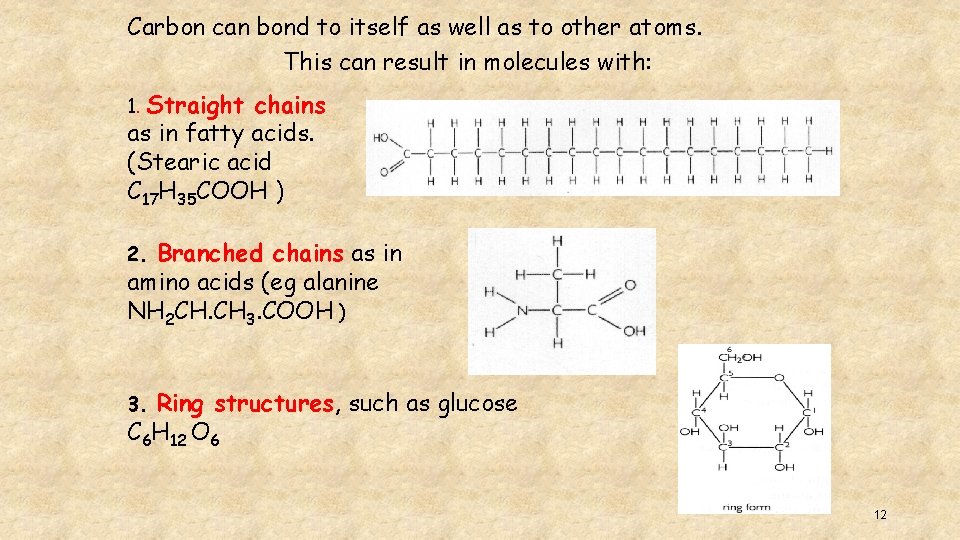
Carbon can bond to itself as well as to other atoms. This can result in molecules with: 1. Straight chains as in fatty acids. (Stearic acid C 17 H 35 COOH ) Branched chains as in amino acids (eg alanine NH 2 CH. CH 3. COOH ) 2. Ring structures, such as glucose C 6 H 12 O 6 3. 12
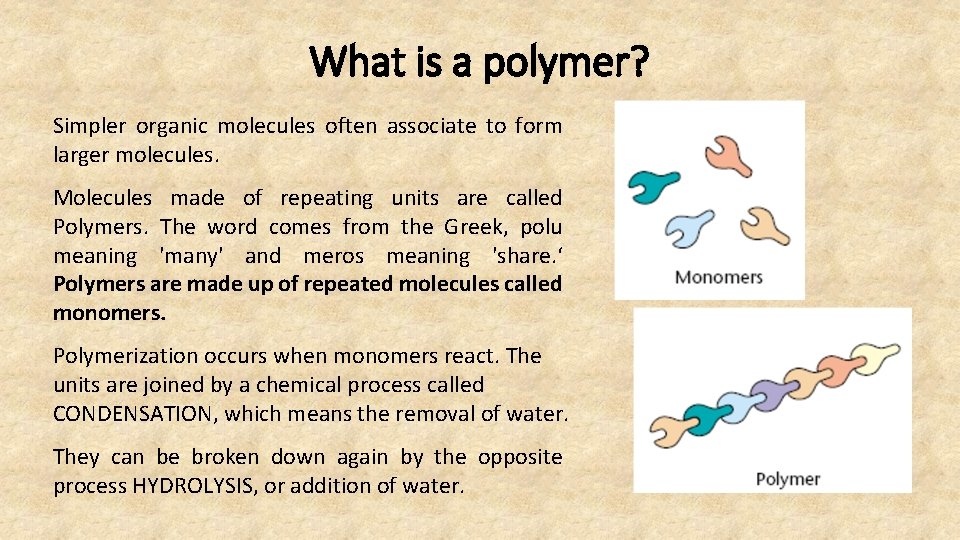
What is a polymer? Simpler organic molecules often associate to form larger molecules. Molecules made of repeating units are called Polymers. The word comes from the Greek, polu meaning 'many' and meros meaning 'share. ‘ Polymers are made up of repeated molecules called monomers. Polymerization occurs when monomers react. The units are joined by a chemical process called CONDENSATION, which means the removal of water. They can be broken down again by the opposite process HYDROLYSIS, or addition of water.
What polymers occur naturally? • DNA, or deoxyribonucleic acid. The DNA molecule is made of monomers called nucleotides. So many nucleotides are linked in a chain to make the DNA polymer molecule. Polynucleotides or nucleic acids are one of three main groups of natural polymers. • Keratin is among the most abundant proteins in humans. It is found in hair and nails. Keratin monomers are amino acids, which form the primary structure of all proteins (polypeptides), a second group of natural polymers. These are made up of monomers called amino acids. • The third type of natural polymers are polysaccharides. These are made of the stuff we have a love-hate relationship with: sugar, or in chemical language, carbohydrate. Animation: http: //www. cengage. com/biology/discipline_content/anim ations/reaction_types. html

Practice Questions: 1. 2. 3. 4. What is a polymer? What is a monomer? Give two examples of monomers Explain what happens in a condensation reaction between two monomers 5. What type of reaction involves the breakage of a chemical bond between two monomers using water? 6. Cytochrome c is a protein used in the reactions of respiration and is found across species of animals, plants and unicellular organisms. Suggest why the widespread occurrence of cytochrome c is considered to be evidence for evolution.

Answers 1. A polymer is a large, complex molecule (long chains) composed of many monomers joined together. 2. A monomer is a small basic molecular unit that can form a polymer. 3. Monosaccharides (e. g. glucose)/amino acids/nucleotides (2) 4. A chemical bond is formed between monomers (1) and a molecule of water is released (1) 5. A hydrolysis reaction. 6. Cytochrome c is present in cells of a wide variety of organisms, suggesting they could all have descended from a common ancestor.

H/W Make a glossary for the following key words Word Meaning Organic molecules Covalent bonds Saturated compounds Unsaturated compounds Monomer Polymerisation Condensation Hydrolysis Hydrogen bond 17

Settling task: In biology certain prefixes are commonly used to indicate numbers. Can you match these Greek/Latin terms to their English translation? • One • Tri • Two • Hexa • Penta • Three • Mono • Four • Poly • Five • Di • Six • Tetra • Many 18

Lesson 3: Carbohydrates • Carbohydrates are substances which contain the elements carbon, hydrogen and oxygen. • They have the general formula Cx(H 2 O)y. • Because hydrogen and oxygen is present in the same proportion as water … they are called carbohydrates which means hydrate of carbon. • There are three main groups; monosaccharides (one sugar), disaccharides and polysaccharides. 30/10/2021 Stretch: All carbs are aldehydes or ketones. Read about aldehydes and ketones here http: //www. chemguide. co. uk/organicprops/carbonyls/background. html 19
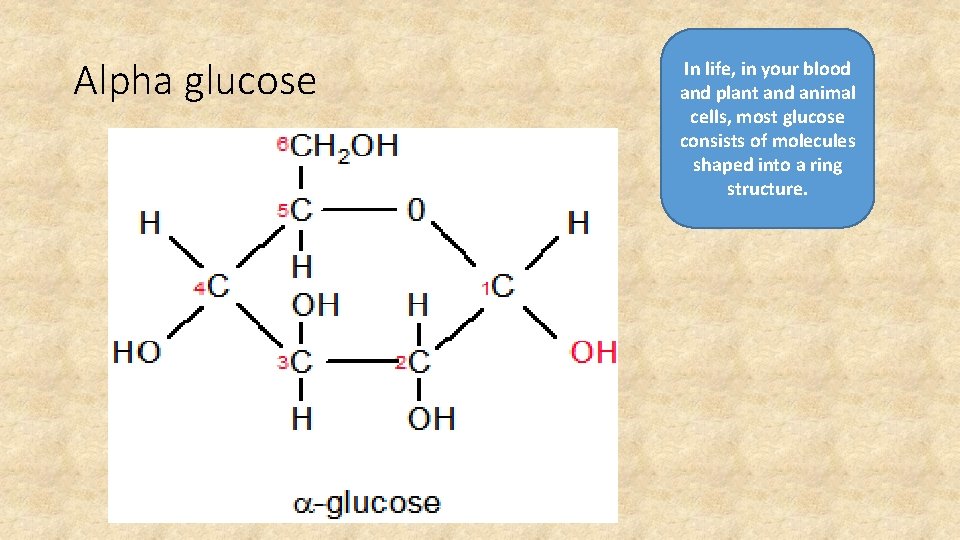
Alpha glucose In life, in your blood and plant and animal cells, most glucose consists of molecules shaped into a ring structure.
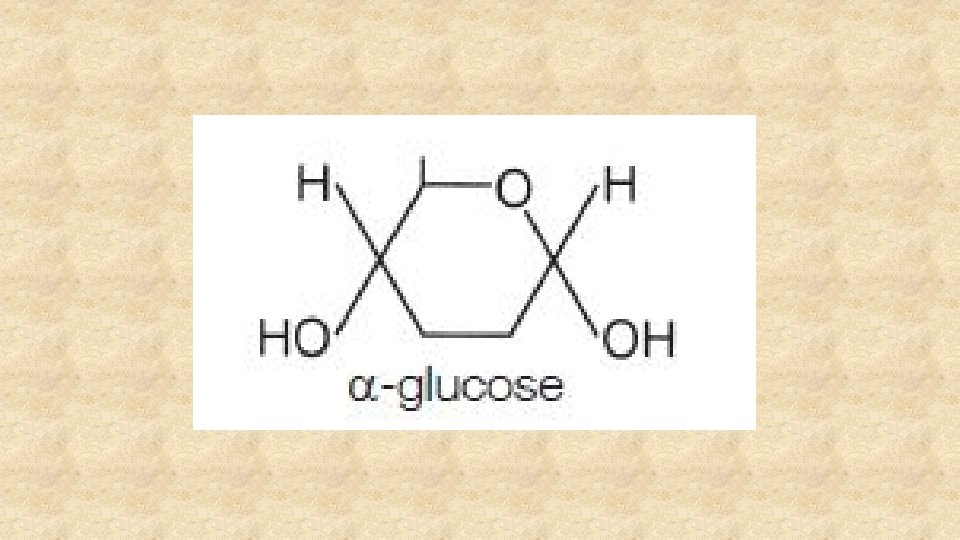
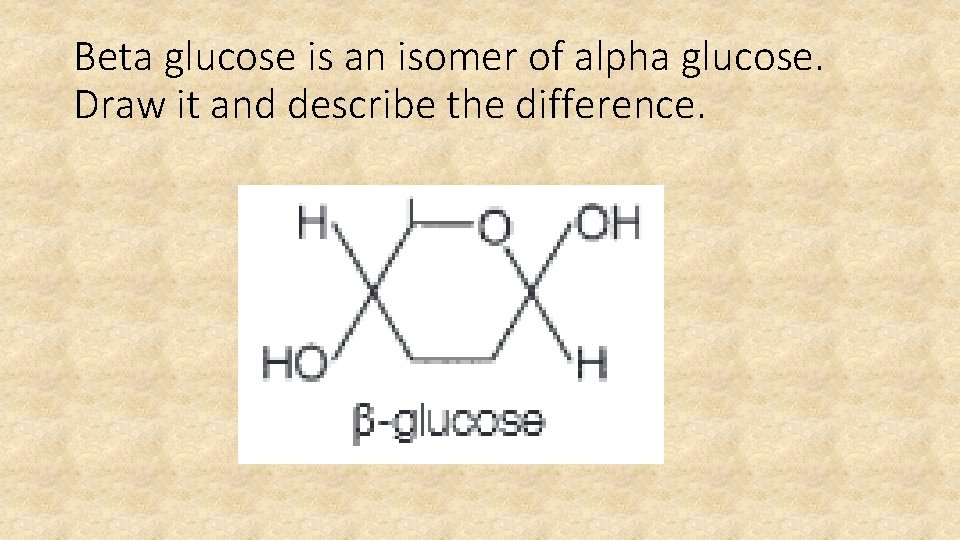
Beta glucose is an isomer of alpha glucose. Draw it and describe the difference.

Monosaccharides are simple sugars • Glucose is important as an energy source in respiration, and as the building block from which starch, glycogen and cellulose are made. • Fructose is also an energy source. It is 200 x sweeter than glucose, and is important industrially as a sweetener in confectionery. With glucose, it is a component of sucrose (cane sugar). • Galactose is important as a component of lactose, the sugar found in milk.
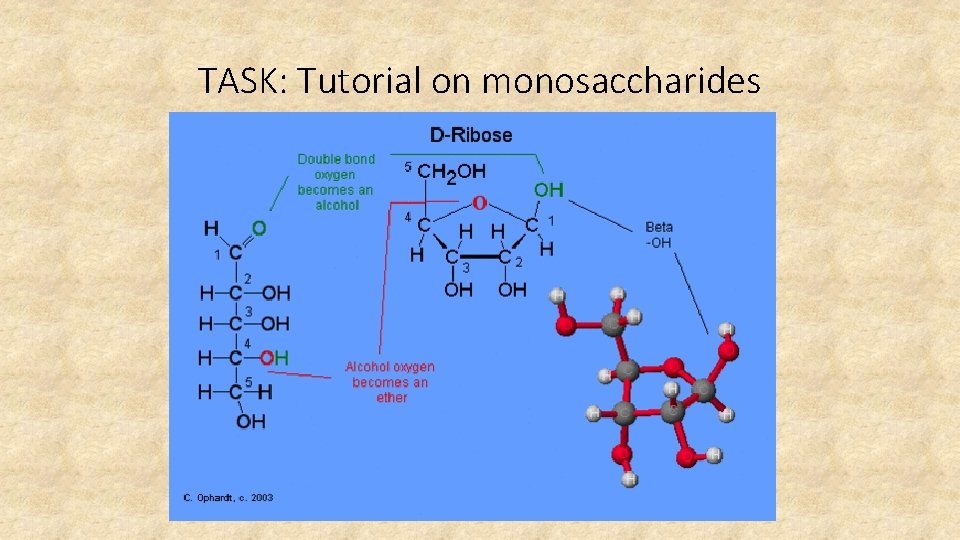
TASK: Tutorial on monosaccharides

Lesson 4 Disaccharides • Monosaccharides are not always suitable for transport and storage. • They are used less quickly if they are linked together to form chains. • Two monosaccharides react together and during the reaction water is eliminated. This is a CONDENSATION REACTION. • The bond between rings is called a GLYCOSIDIC BOND. • This forms a DISACCHARIDE
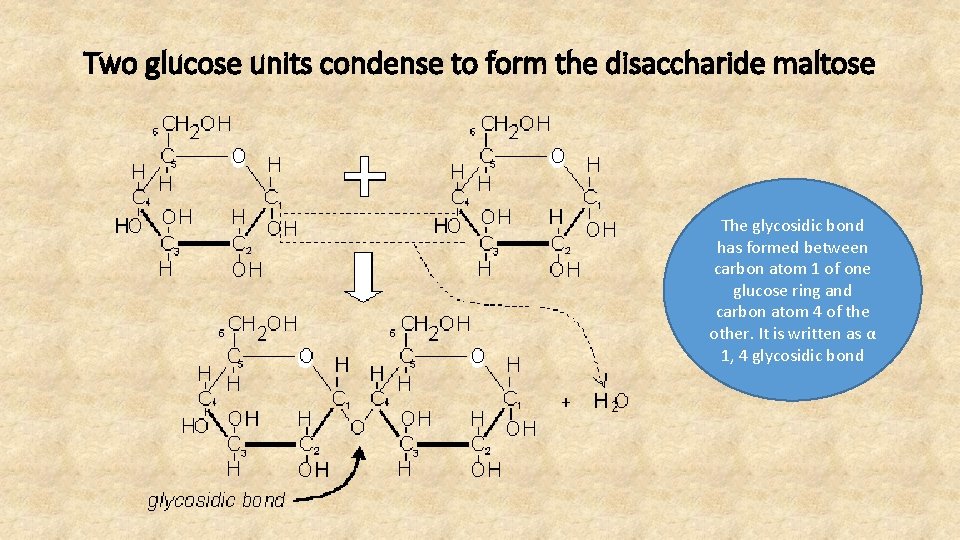
Two glucose units condense to form the disaccharide maltose The glycosidic bond has formed between carbon atom 1 of one glucose ring and carbon atom 4 of the other. It is written as α 1, 4 glycosidic bond

Do you understand monosaccharides and disaccharides? 1. Read pages 20 -21 Answer both application questions and summary questions. 2. Read page 22. Answer questions 1 and 2. PLENARY: https: //www. youtube. com/watch? v=_zm_Dy. D 6 FJ 0 H/W: Complete the worksheet on designing an experiment. Due next Friday.
Series 1 0, 8 0, 7 0, 6 0, 5 0, 4 0, 3 0, 2 0, 1 0 0 0, 05 0, 15 0, 25 0, 35
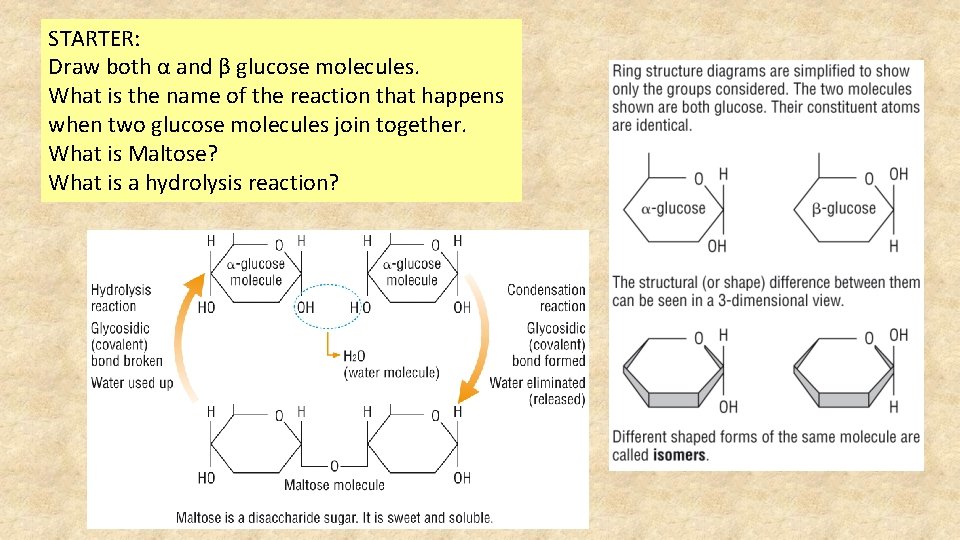
STARTER: Draw both α and β glucose molecules. What is the name of the reaction that happens when two glucose molecules join together. What is Maltose? What is a hydrolysis reaction?
Polysaccharides • Formed when many hundreds of monosaccharide units condense to from chains. • They are usually very long; • Branched or unbranched • Folded – this makes them compact and good for storage e. g. starch or glycogen • Straight or coiled – this can be meshed and is ideal for construction e. g. cellulose THE KEY POLYSACCHARIDES WE NEED TO KNOW ARE STARCH, GLYCOGEN AND CELLULOSE All 3 are insoluble in water and therefore have no osmotic effect. They cannot diffuse out of the cell. TASK: Write down everything you already know about starch, glycogen and cellulose.
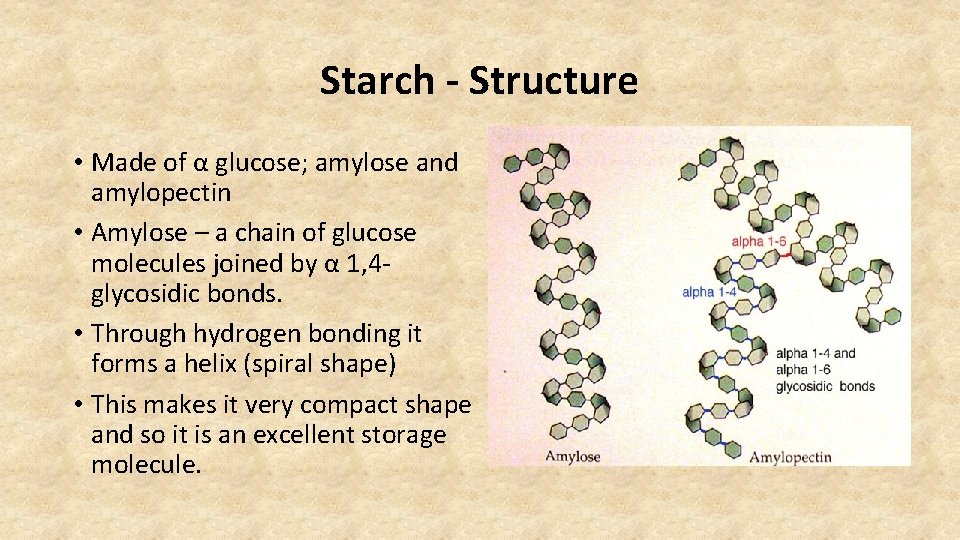
Starch - Structure • Made of α glucose; amylose and amylopectin • Amylose – a chain of glucose molecules joined by α 1, 4 glycosidic bonds. • Through hydrogen bonding it forms a helix (spiral shape) • This makes it very compact shape and so it is an excellent storage molecule.
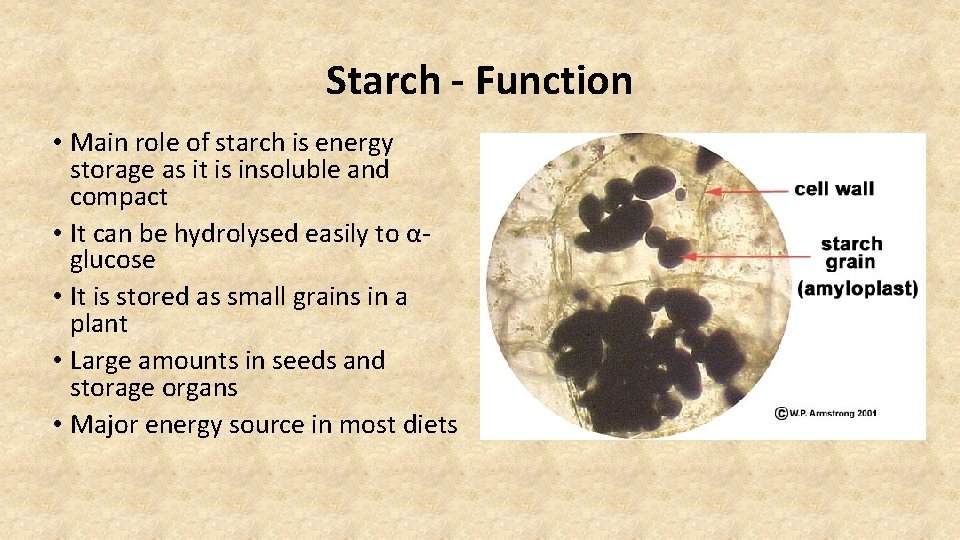
Starch - Function • Main role of starch is energy storage as it is insoluble and compact • It can be hydrolysed easily to αglucose • It is stored as small grains in a plant • Large amounts in seeds and storage organs • Major energy source in most diets
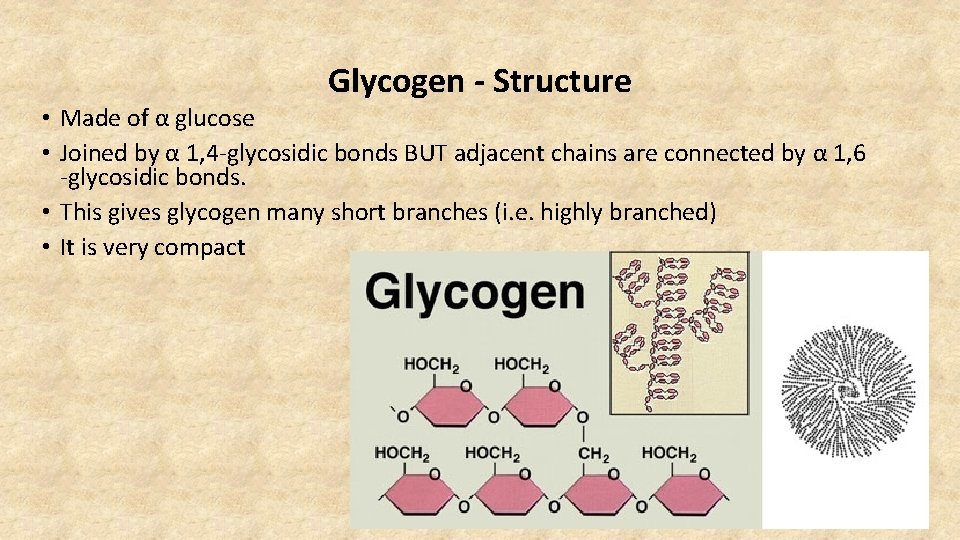
Glycogen - Structure • Made of α glucose • Joined by α 1, 4 -glycosidic bonds BUT adjacent chains are connected by α 1, 6 -glycosidic bonds. • This gives glycogen many short branches (i. e. highly branched) • It is very compact
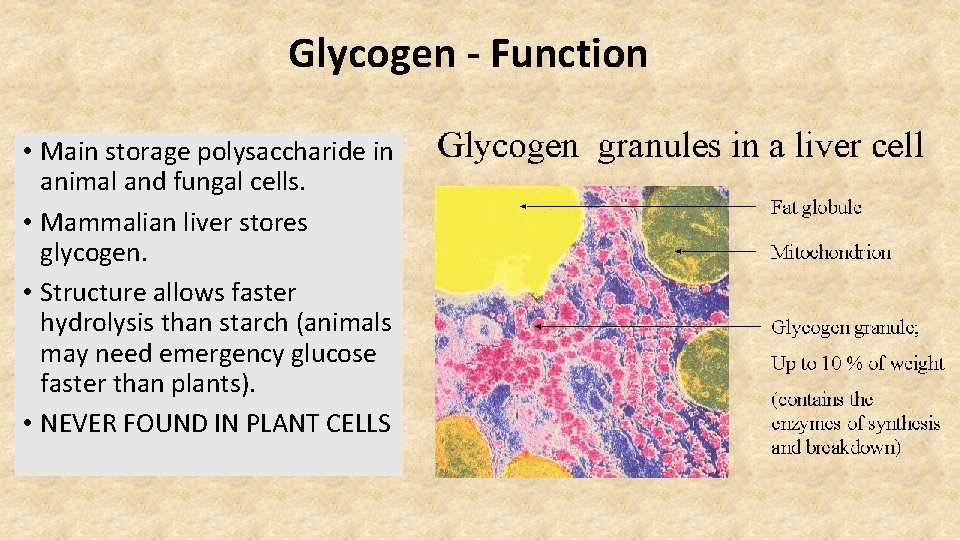
Glycogen - Function • Main storage polysaccharide in animal and fungal cells. • Mammalian liver stores glycogen. • Structure allows faster hydrolysis than starch (animals may need emergency glucose faster than plants). • NEVER FOUND IN PLANT CELLS

Activity - Exam Style Questions 1. Glycogen and protein are both polymers. Explain why there is only one type of glucose but several types of protein. (2) 2. A starch molecule has a spiral shape. Explain why this shape is important to its function in cells. (1) 3. What is the difference in chemical bonding between amylose and glycogen? (2) 4. i) Name the monomer present in starch (1) ii) Name the type of reaction that converts starch to its monomers (1) 5. Draw a β glucose molecule (2)

Starter: For each of these statements, pick the correct carbohydrate from the list opposite 1. Stains deep blue with iodine solution 2. Is known as ‘animal starch’ 3. Found in plants 4. Are polysaccharides 5. Monosaccharide found in starch 6. Has a structural function 7. Can be hydrolysed 8. Easily diffuses in and out of cells Carbohydrates – may be used once, more than once or not at all • α-glucose • β-glucose • Starch • Cellulose • Glycogen

Carbohydrates - Answers 1. Stains deep blue with iodine solution - Starch 2. Is known as ‘animal starch’ - Glycogen 3. Found in plants - α-glucose, β-glucose, starch, cellulose 4. Are polysaccharides - Starch, cellulose, glycogen 5. Monosaccharide found in starch - α-glucose 6. Has a structural function - Cellulose 7. Can be hydrolysed - Starch, cellulose, glycogen 8. Easily diffuses in and out of cells - α-glucose, βglucose
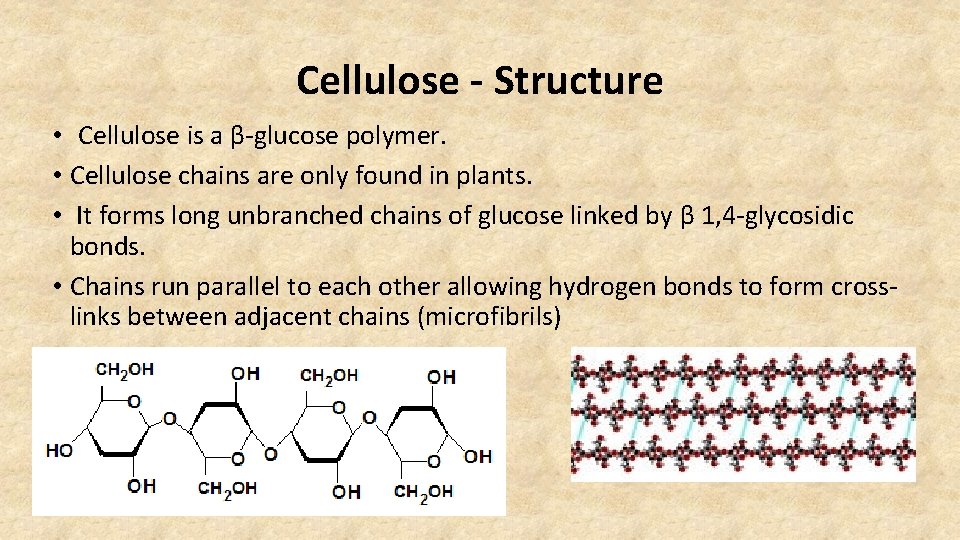
Cellulose - Structure • Cellulose is a β-glucose polymer. • Cellulose chains are only found in plants. • It forms long unbranched chains of glucose linked by β 1, 4 -glycosidic bonds. • Chains run parallel to each other allowing hydrogen bonds to form crosslinks between adjacent chains (microfibrils)
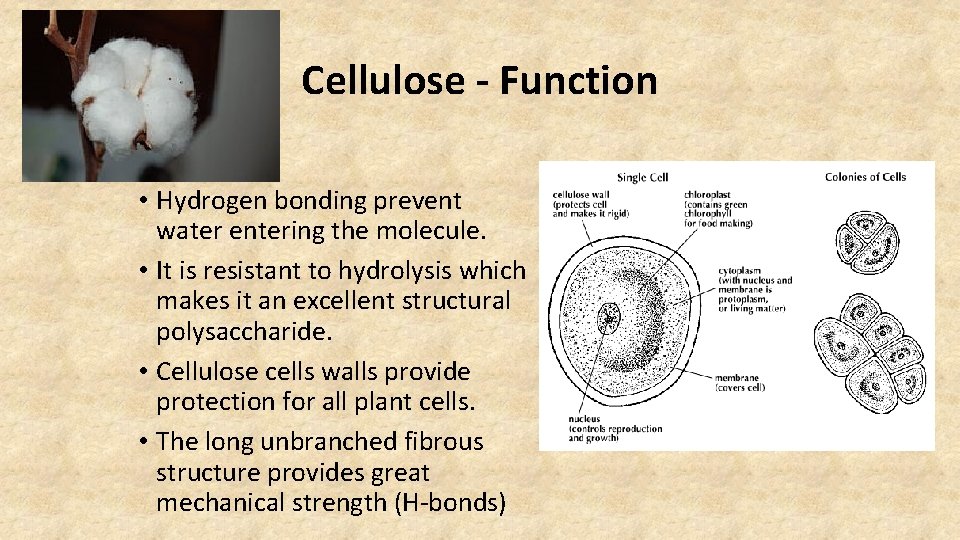
Cellulose - Function • Hydrogen bonding prevent water entering the molecule. • It is resistant to hydrolysis which makes it an excellent structural polysaccharide. • Cellulose cells walls provide protection for all plant cells. • The long unbranched fibrous structure provides great mechanical strength (H-bonds)
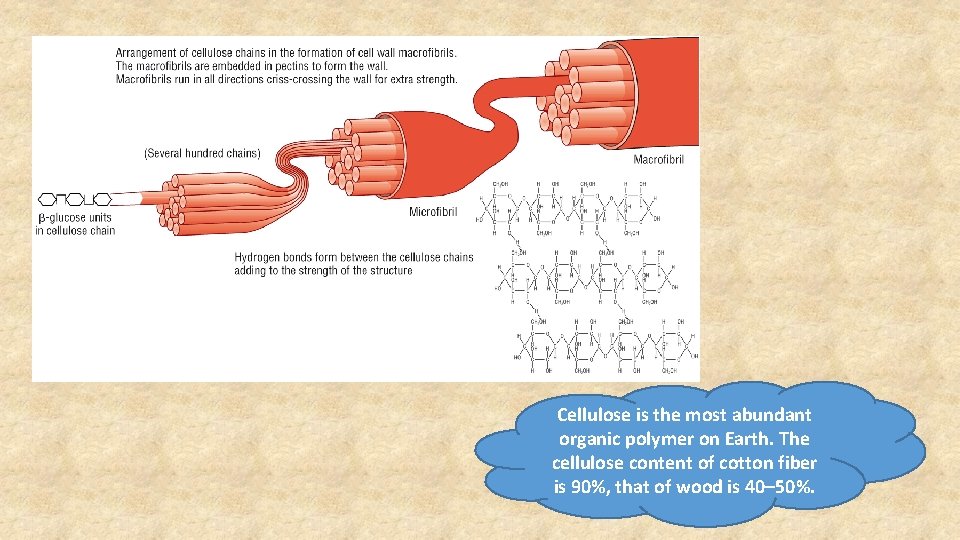
Cellulose is the most abundant organic polymer on Earth. The cellulose content of cotton fiber is 90%, that of wood is 40– 50%.

Activity: Complete the exam questions on carbohydrates

The term lipid is applied to a range of more or less unrelated biological molecules, whose only common property is their HYDROPHOBIC nature: in general lipids dissolve easily in organic solvents such as propanone, but poorly or not at all in water. What is the difference between a fat and an oil? LIPIDS

TRUE/FALSE • Fats are liquid at room temperature • Oils are liquid at room temperature • Fats and oils are mostly TRIGLYCERIDES • A triglyceride is composed of two fatty acids and a four-carbon molecule glycerol. • Saturated means it has double bonds • Unsaturated means it has double bonds • There is a glycosidic bond between glycerol and fatty acid
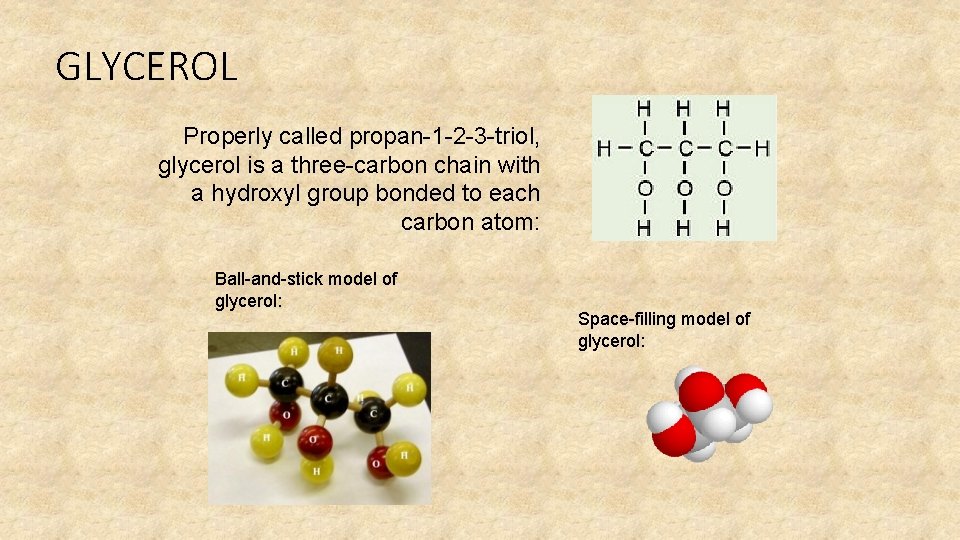
GLYCEROL Properly called propan-1 -2 -3 -triol, glycerol is a three-carbon chain with a hydroxyl group bonded to each carbon atom: Ball-and-stick model of glycerol: Space-filling model of glycerol:
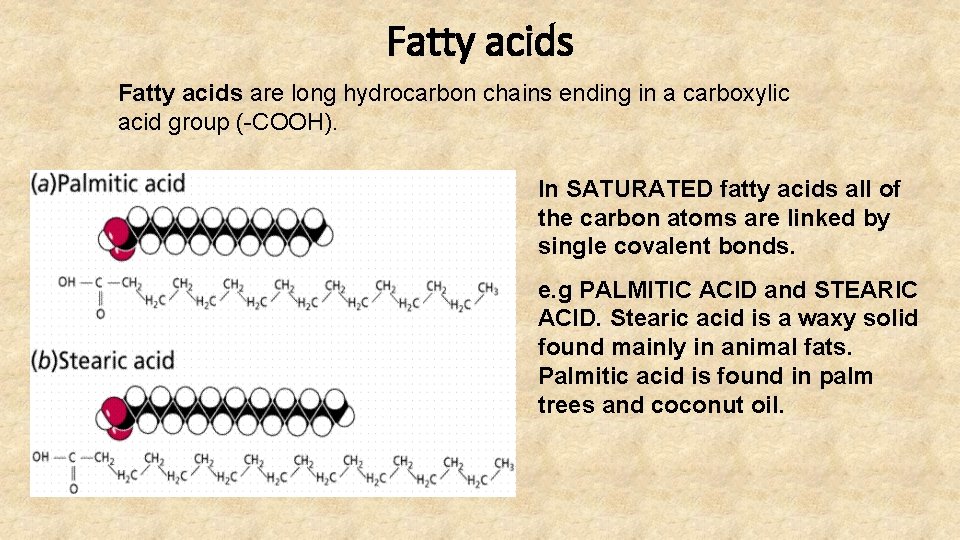
Fatty acids are long hydrocarbon chains ending in a carboxylic acid group (-COOH). In SATURATED fatty acids all of the carbon atoms are linked by single covalent bonds. e. g PALMITIC ACID and STEARIC ACID. Stearic acid is a waxy solid found mainly in animal fats. Palmitic acid is found in palm trees and coconut oil.
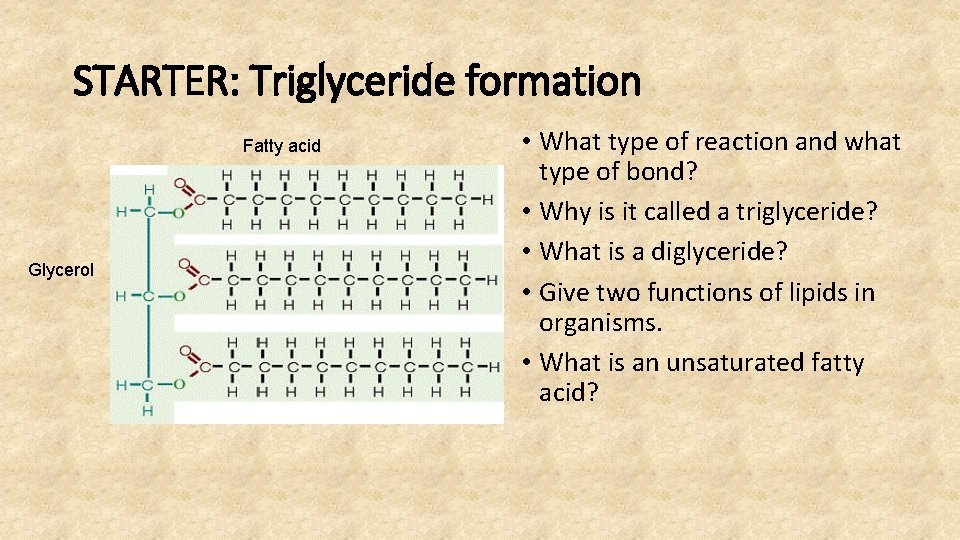
STARTER: Triglyceride formation Fatty acid Glycerol • What type of reaction and what type of bond? • Why is it called a triglyceride? • What is a diglyceride? • Give two functions of lipids in organisms. • What is an unsaturated fatty acid?

A simplified diagram
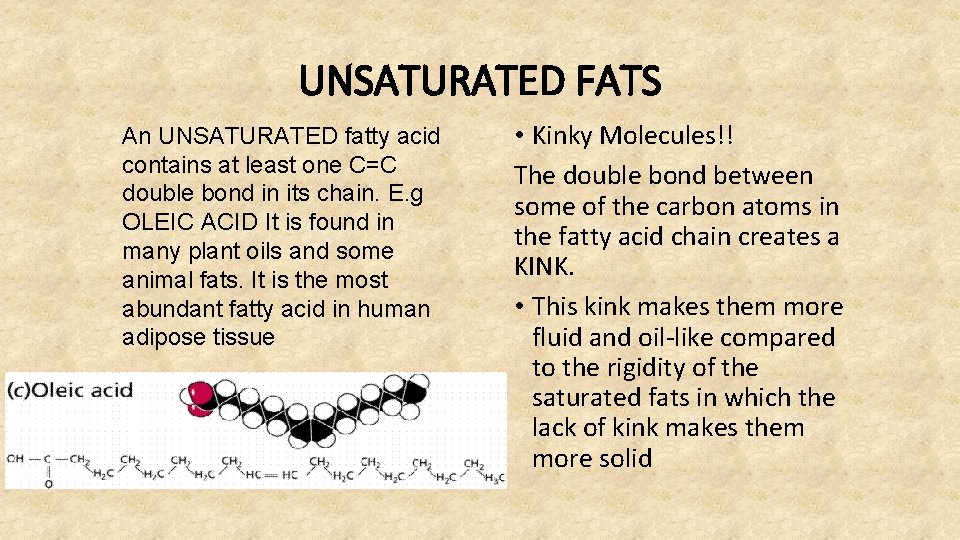
UNSATURATED FATS An UNSATURATED fatty acid contains at least one C=C double bond in its chain. E. g OLEIC ACID It is found in many plant oils and some animal fats. It is the most abundant fatty acid in human adipose tissue • Kinky Molecules!! The double bond between some of the carbon atoms in the fatty acid chain creates a KINK. • This kink makes them more fluid and oil-like compared to the rigidity of the saturated fats in which the lack of kink makes them more solid
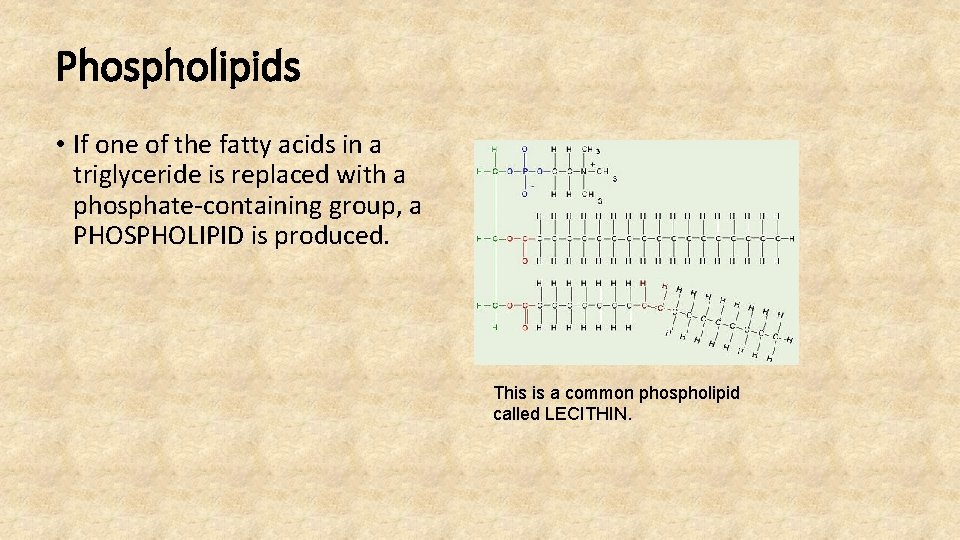
Phospholipids • If one of the fatty acids in a triglyceride is replaced with a phosphate-containing group, a PHOSPHOLIPID is produced. This is a common phospholipid called LECITHIN.
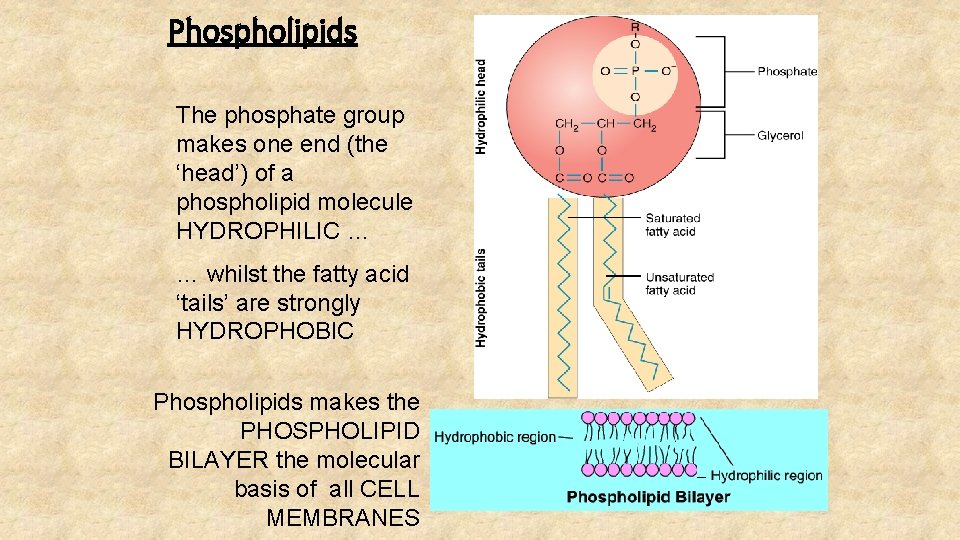
Phospholipids The phosphate group makes one end (the ‘head’) of a phospholipid molecule HYDROPHILIC … … whilst the fatty acid ‘tails’ are strongly HYDROPHOBIC Phospholipids makes the PHOSPHOLIPID BILAYER the molecular basis of all CELL MEMBRANES
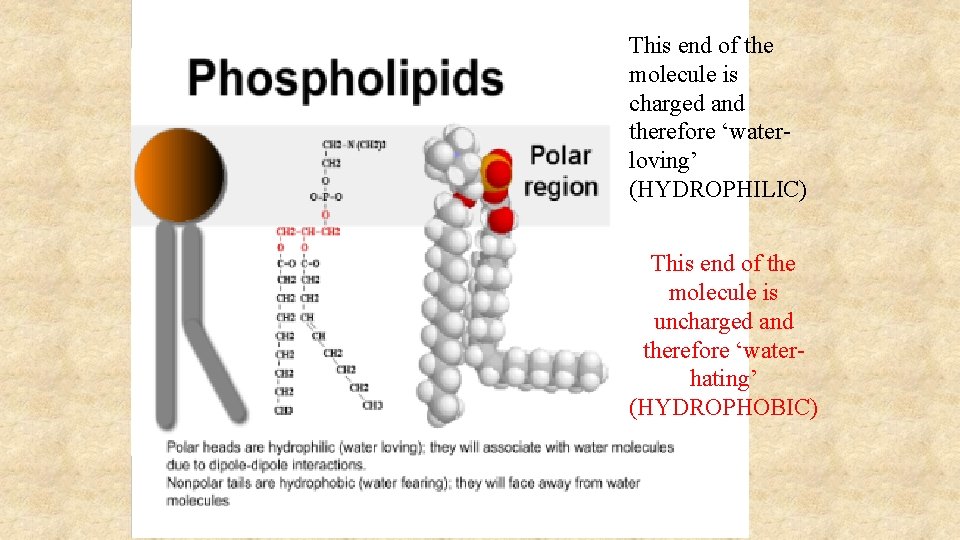
This end of the molecule is charged and therefore ‘waterloving’ (HYDROPHILIC) This end of the molecule is uncharged and therefore ‘waterhating’ (HYDROPHOBIC)
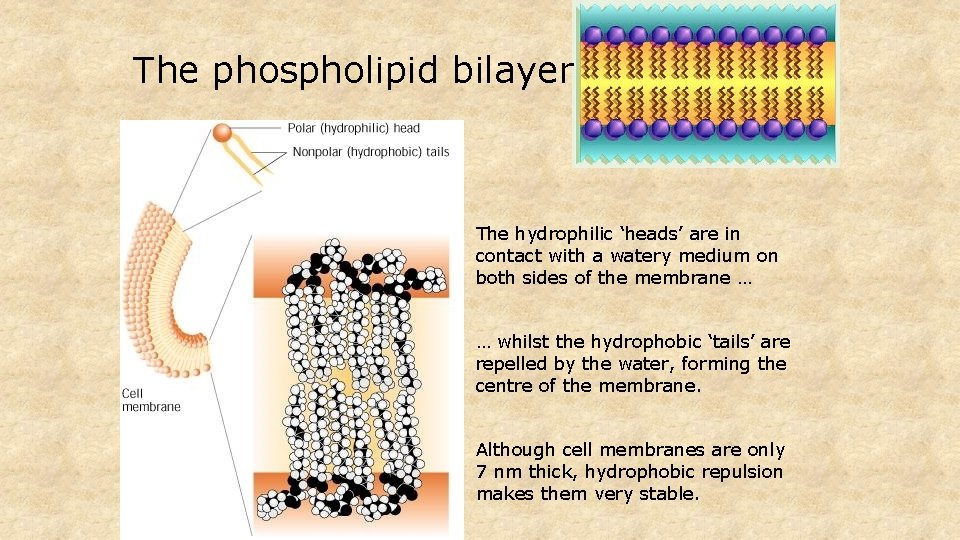
The phospholipid bilayer The hydrophilic ‘heads’ are in contact with a watery medium on both sides of the membrane … … whilst the hydrophobic ‘tails’ are repelled by the water, forming the centre of the membrane. Although cell membranes are only 7 nm thick, hydrophobic repulsion makes them very stable.

What your specification says ……… • Triglycerides and phospholipids are two groups of lipid. • Triglycerides are formed by the condensation of one molecule of glycerol and three molecules of fatty acid. • A condensation reaction between glycerol and a fatty acid (RCOOH) forms an ester bond. • The R-group of a fatty acid may be saturated or unsaturated. • The different properties of triglycerides related to their structures.

Your specification ……………… Triglycerides and phospholipids are two groups of lipid. In phospholipids, one of the fatty acids of a triglyceride is substituted by a phosphate-containing group. The different properties of triglycerides and phospholipids related to their different structures. Students should be able to: recognise, from diagrams, saturated and unsaturated fatty acids explain the different properties of triglycerides and phospholipids.
- Slides: 56