Unit 1 2 Carbon and the Molecular Diversity

Unit 1. 2 Carbon and the Molecular Diversity of Life 1
2
Carbon Chemistry 1. Carbon is the Backbone of proteins, lipids, carbohydrates and nucleic acids (macromolecules) Figure 4. 1 3

Carbon Chemistry 1. Carbon is the Backbone of proteins, lipids, carbohydrates and nucleic acids (macromolecules) 2. All living organisms are made up of these macromolecules made mostly of carbon 4

Carbon Chemistry 3. Carbon atoms form many molecules by bonding to four other atoms 5
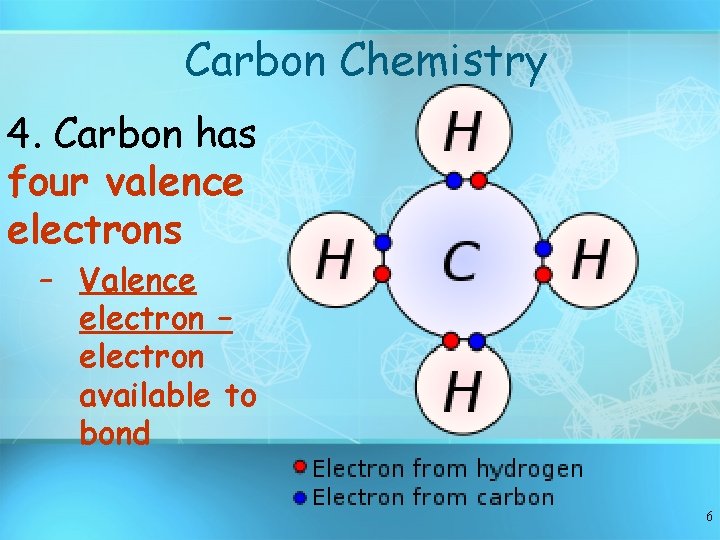
Carbon Chemistry 4. Carbon has four valence electrons – Valence electron – electron available to bond 6
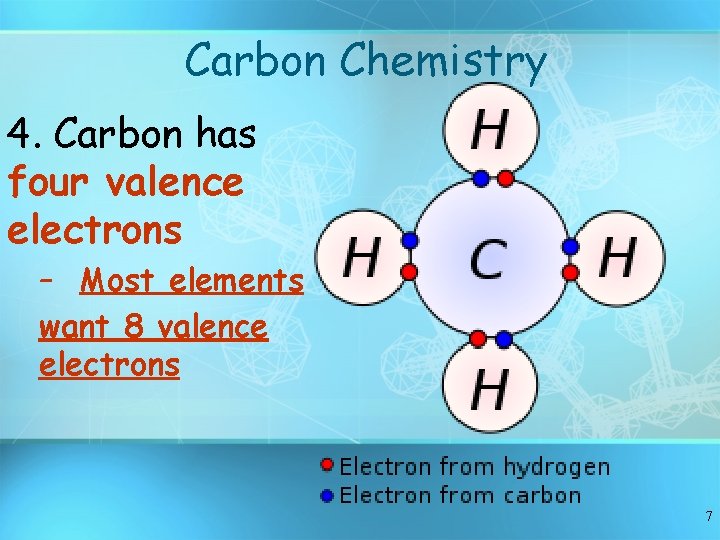
Carbon Chemistry 4. Carbon has four valence electrons – Most elements want 8 valence electrons 7
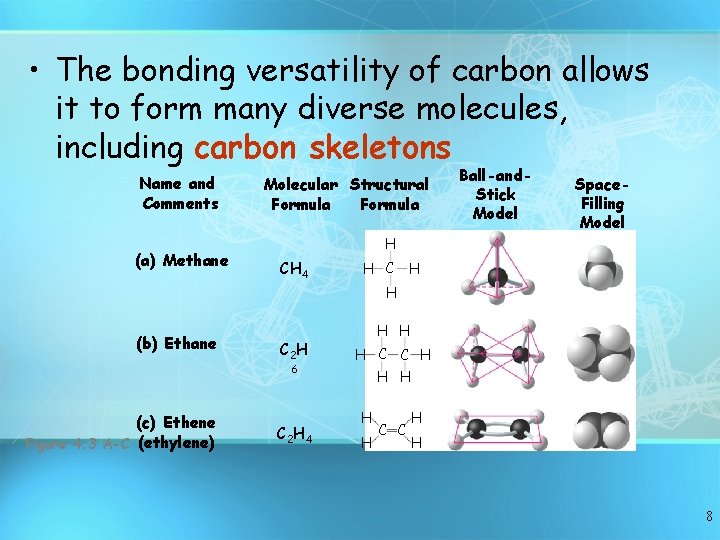
• The bonding versatility of carbon allows it to form many diverse molecules, including carbon skeletons Name and Comments (a) Methane Molecular Structural Formula Ball-and. Stick Model Space. Filling Model H CH 4 H C H H (b) Ethane C 2 H 6 (c) Ethene Figure 4. 3 A-C (ethylene) C 2 H 4 H H H C C H H 8
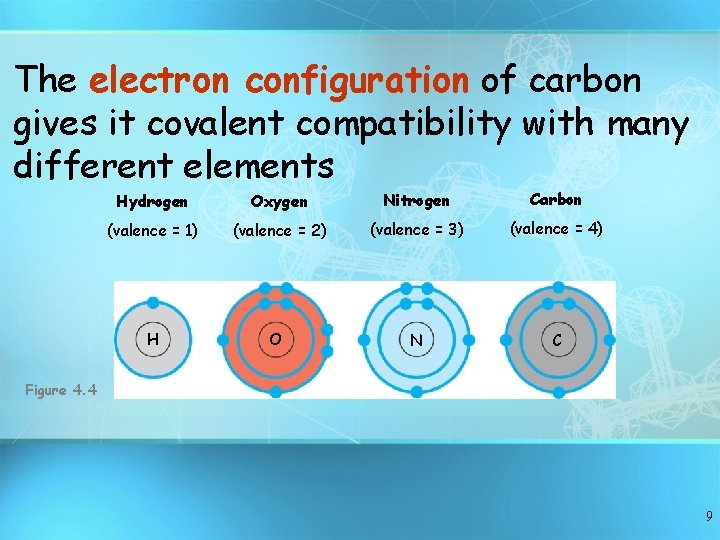
The electron configuration of carbon gives it covalent compatibility with many different elements Hydrogen Oxygen Nitrogen Carbon (valence = 1) (valence = 2) (valence = 3) (valence = 4) H O N C Figure 4. 4 9
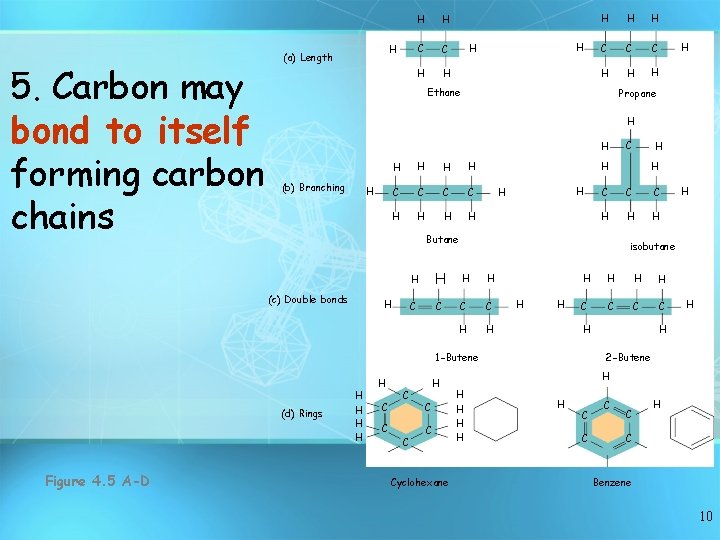
5. Carbon may bond to itself forming carbon chains H (a) Length H H C C H H H C C C H H H Ethane H Propane H H (b) Branching H H H C C H H H C C H H (d) Rings H H C C Cyclohexane H H C C C H H isobutane H H H C C H 1 -Butene H C H Butane (c) Double bonds Figure 4. 5 A-D H H H 2 -Butene H H H C C H C Benzene 10
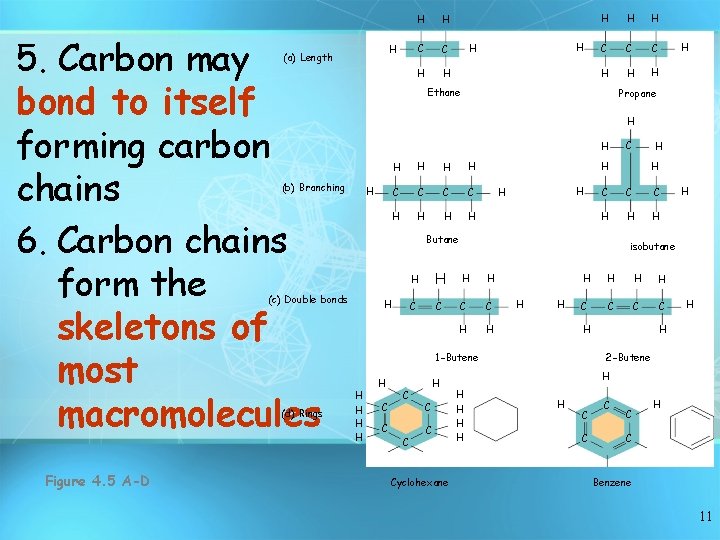
5. Carbon may bond to itself forming carbon chains 6. Carbon chains form the skeletons of most macromolecules H (a) Length C C H H H H C C C H H Propane H H H H C C C C H H H C C H C C Cyclohexane H H C C C H H isobutane H H H C C H 1 -Butene H C H Butane (c) Double bonds Figure 4. 5 A-D H Ethane (b) Branching (d) Rings H H H 2 -Butene H H H C C H C Benzene 11
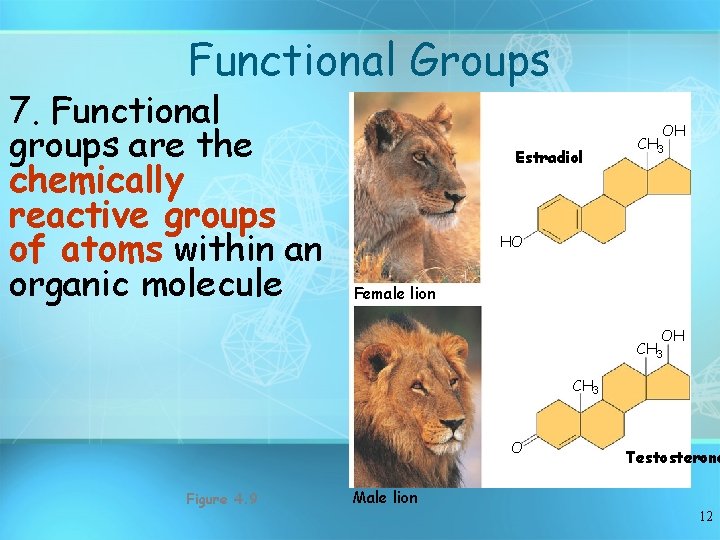
Functional Groups 7. Functional groups are the chemically reactive groups of atoms within an organic molecule Estradiol OH CH 3 HO Female lion OH CH 3 O Figure 4. 9 Testosterone Male lion 12
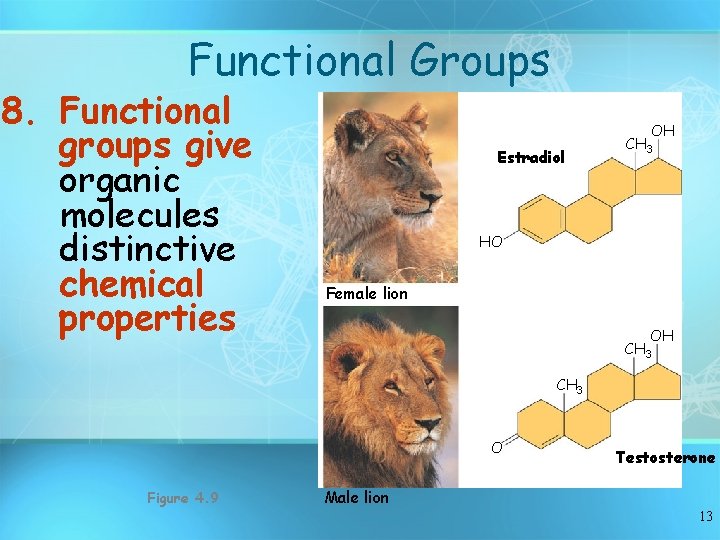
Functional Groups 8. Functional groups give organic molecules distinctive chemical properties Estradiol OH CH 3 HO Female lion OH CH 3 O Figure 4. 9 Testosterone Male lion 13
• Six functional groups are important in the chemistry of life – Hydroxyl – Carbonyl – Carboxyl – Amino – Sulfhydryl – Phosphate 14
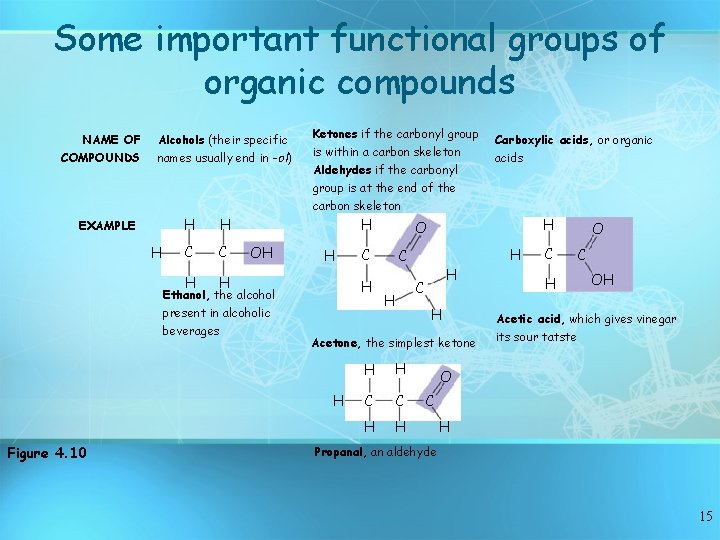
Some important functional groups of organic compounds NAME OF COMPOUNDS Alcohols (their specific names usually end in -ol) EXAMPLE H H H C C H H Ketones if the carbonyl group is within a carbon skeleton Aldehydes if the carbonyl group is at the end of the carbon skeleton H OH Ethanol, the alcohol present in alcoholic beverages H Figure 4. 10 H H C H H Acetone, the simplest ketone H acids O C H Carboxylic acids, or organic H H C C H H C H O C OH Acetic acid, which gives vinegar its sour tatste O C H Propanal, an aldehyde 15
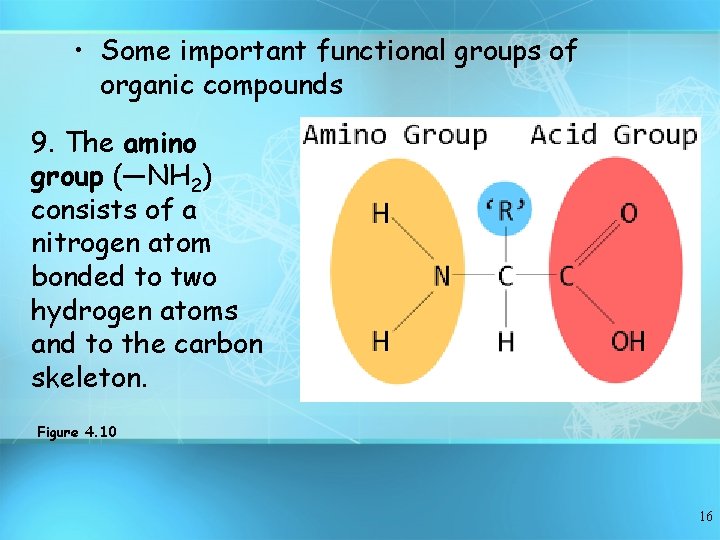
• Some important functional groups of organic compounds 9. The amino group (—NH 2) consists of a nitrogen atom bonded to two hydrogen atoms and to the carbon skeleton. Figure 4. 10 16
- Slides: 16