Unigold Recombigen HIV 12 Training for HIV Testing

Unigold Recombigen HIV 1/2 Training for HIV Testing Sites Updated: February 2018 Cicely Richard Office of HIV/AIDS

Updated: February 2018 Cicely Richard Office of HIV/AIDS UNIGOLD RECOMBIGEN HIV 1/2 TRAINING FOR HIV TESTING SITES

Objective • The objective of today’s training is to become familiar with the Unigold Recombigen HIV 1/2 test. This is a 3 rd generation HIV antibody test. This testing device is currently utilized only by HIV testing sites that are approved to use the DPH rapid/rapid testing algorithm.

CLINICAL CHEMISTRY | COAGULATION | INFECTIOUS DISEASE | POINT OF CARE Uni-Gold™ Recombigen HIV 1/2 Training Lou Pastors MT(ASCP National Account Manager Trinity Biotech Phone: 630 -561 -6681 e. Mail: louis. pastors@trinityusa. com 4

Uni-Gold™ Recombigen® HIV NAME AND INTENDED USE Uni-Gold™ Recombigen® HIV is a single use rapid immunoassay for the qualitative detection of antibodies to HIV-1 and /or HIV-2 in serum, plasma and whole blood (venipuncture and fingerstick). Uni-Gold™ Recombigen® HIV is intended for use in point of care settings as an aid in diagnosis of infection with HIV-1 and HIV-2. * Uni-Gold™ Recombigen® HIV Package Insert, pg. 1 5
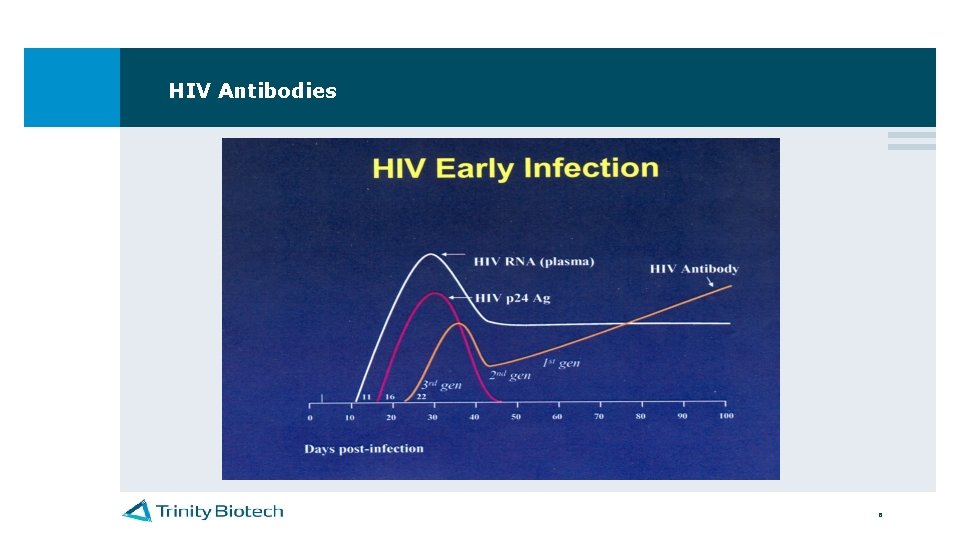
HIV Antibodies 6
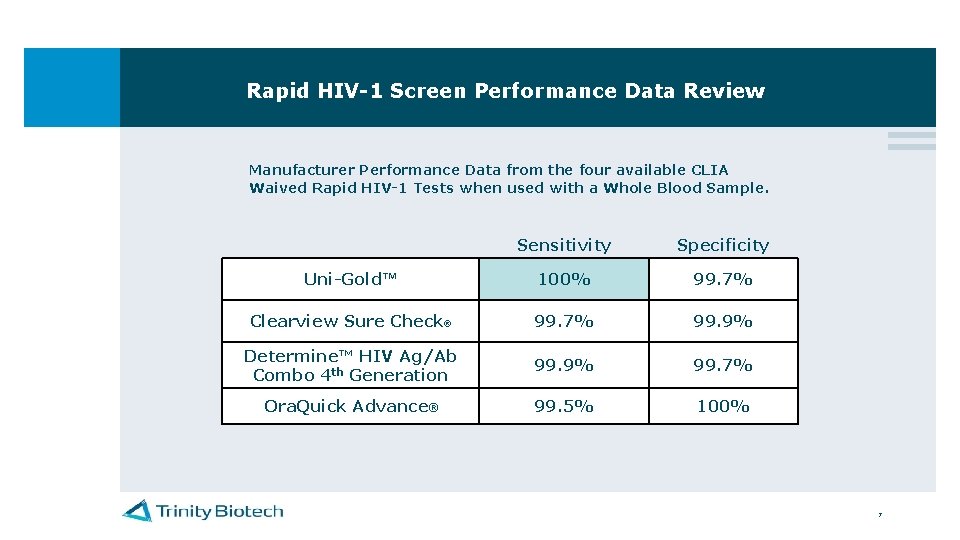
Rapid HIV-1 Screen Performance Data Review Manufacturer Performance Data from the four available CLIA Waived Rapid HIV-1 Tests when used with a Whole Blood Sample. Sensitivity Specificity Uni-Gold™ 100% 99. 7% Clearview Sure Check® 99. 7% 99. 9% Determine™ HIV Ag/Ab Combo 4 th Generation 99. 9% 99. 7% Ora. Quick Advance® 99. 5% 100% 7
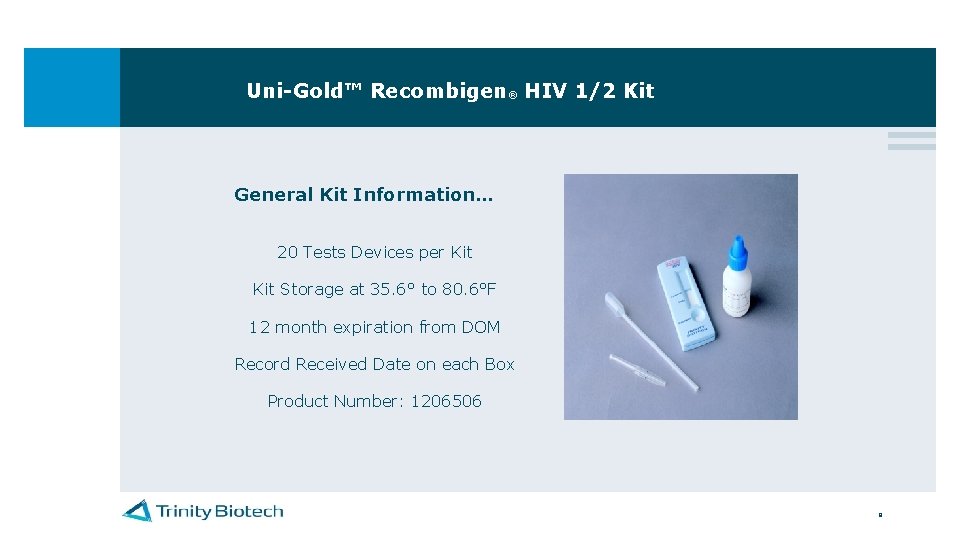
Uni-Gold™ Recombigen® HIV 1/2 Kit General Kit Information… 20 Tests Devices per Kit Storage at 35. 6° to 80. 6°F 12 month expiration from DOM Record Received Date on each Box Product Number: 1206506 8
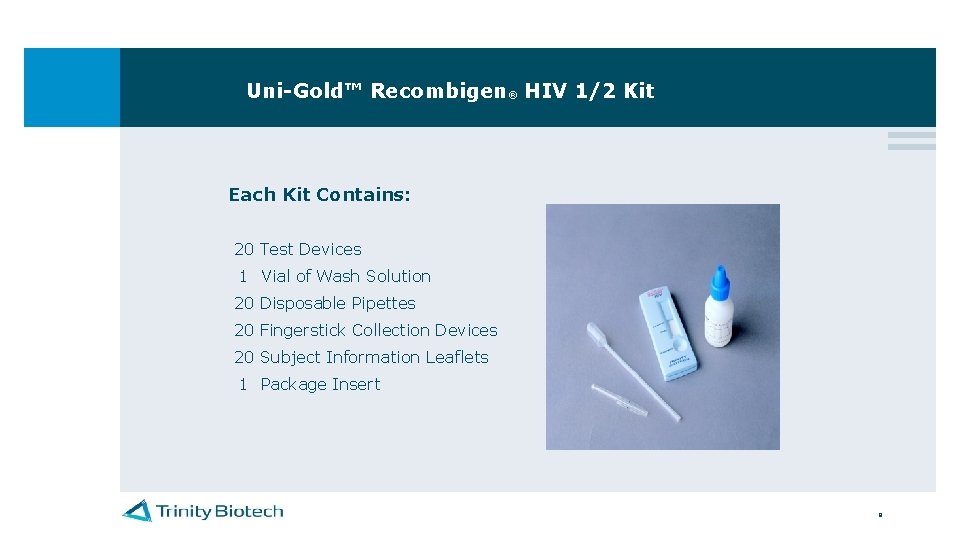
Uni-Gold™ Recombigen® HIV 1/2 Kit Each Kit Contains: 20 Test Devices 1 Vial of Wash Solution 20 Disposable Pipettes 20 Fingerstick Collection Devices 20 Subject Information Leaflets 1 Package Insert 9
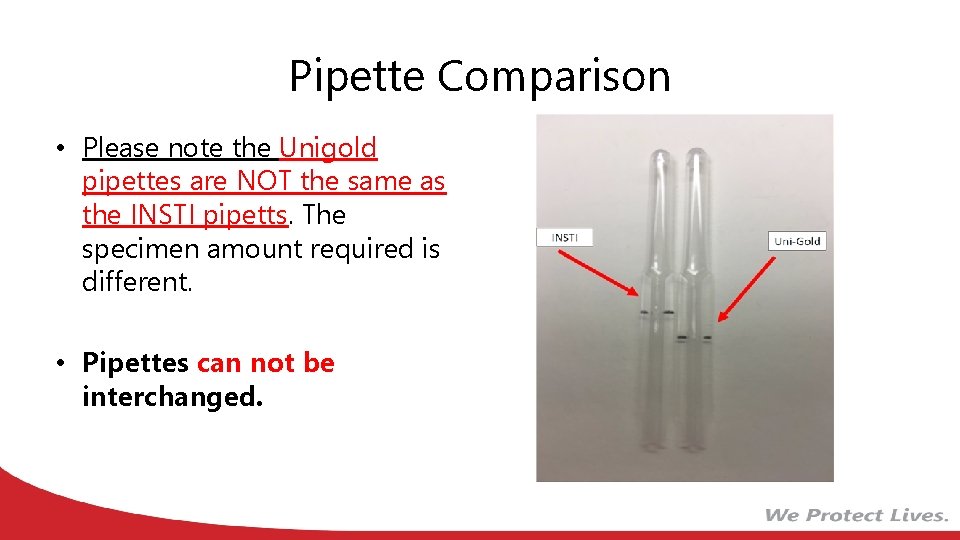
Pipette Comparison • Please note the Unigold pipettes are NOT the same as the INSTI pipetts. The specimen amount required is different. • Pipettes can not be interchanged.

Uni-Gold™ Recombigen® HIV 1/2 Controls Each Set of Controls Contains: • 1 Vial of Positive HIV-1 Control – O. 5 m. L • 1 Vial of Positive HIV-2 Control – 0. 5 m. L • 1 Vial of Negative HIV Control – 0. 5 m. L • 1 Package Insert Always Store at 2⁰ to 8⁰ C 12 Month Expiration from DOM Open Vial Stability is One Month Product Number: 1206530 11

Uni-Gold™ Rapid HIV Fingerstick Procedure Materials required for Uni-Gold™ Fingerstick Procedure – – – Uni-Gold™ Test Device (Room Temp, 20 Minutes stored at 2 -8° C) Uni-Gold™ Wash Solution (blue top dropper provided in Kit) Uni-Gold™ Fingerstick Collection Device (provided in Kit) Uni-Gold™ Subject Information Leaflet (provided in Kit) Lancet* (Any Blade Lancet Capable of a 50 µl Bleed) Sterile Gauze*, Alcohol Wipe*, and Band Aid* Latex Gloves* Marker/Sharpie* Biohazard Container* Sharps Container* Timer* (Timers provided by Trinity Biotech upon request) *Materials required but not provided with Uni-Gold™ Kit 12

Uni-Gold™ Rapid HIV Fingerstick Procedure Organize Materials required for Procedure Open a new Test Device and mark with Patient ID 13
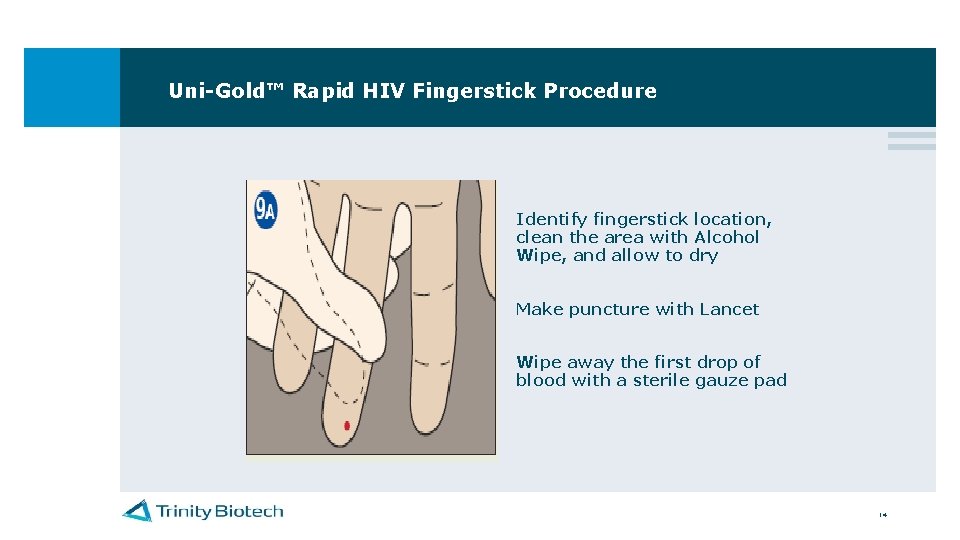
Uni-Gold™ Rapid HIV Fingerstick Procedure Identify fingerstick location, clean the area with Alcohol Wipe, and allow to dry Make puncture with Lancet Wipe away the first drop of blood with a sterile gauze pad 14
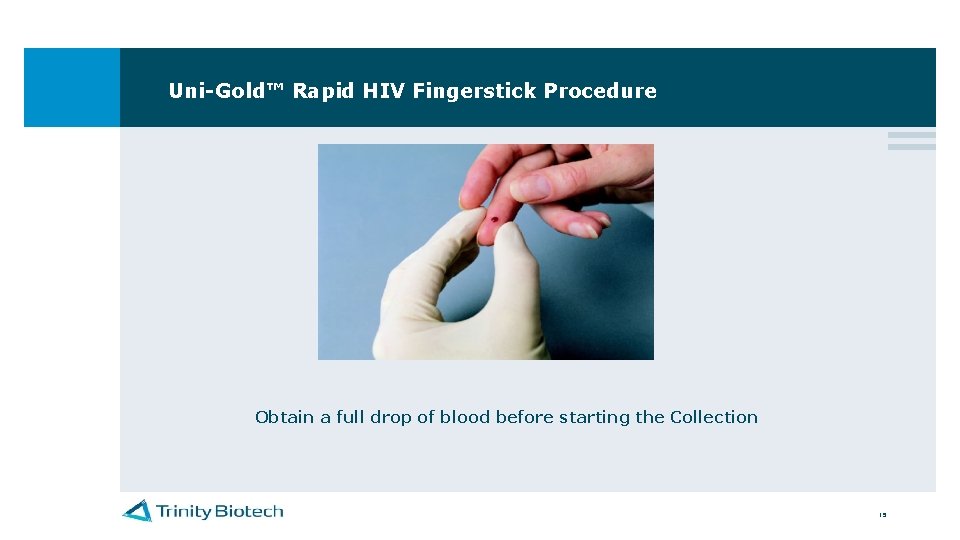
Uni-Gold™ Rapid HIV Fingerstick Procedure Obtain a full drop of blood before starting the Collection 15

Uni-Gold™ Rapid HIV Fingerstick Procedure Holding the Fingerstick Collection Device horizontally Touch the tip of the Collection Device to the sample surface Fill the Fingerstick Collection Device to the black line Do NOT squeeze the bulb to collect the sample 16
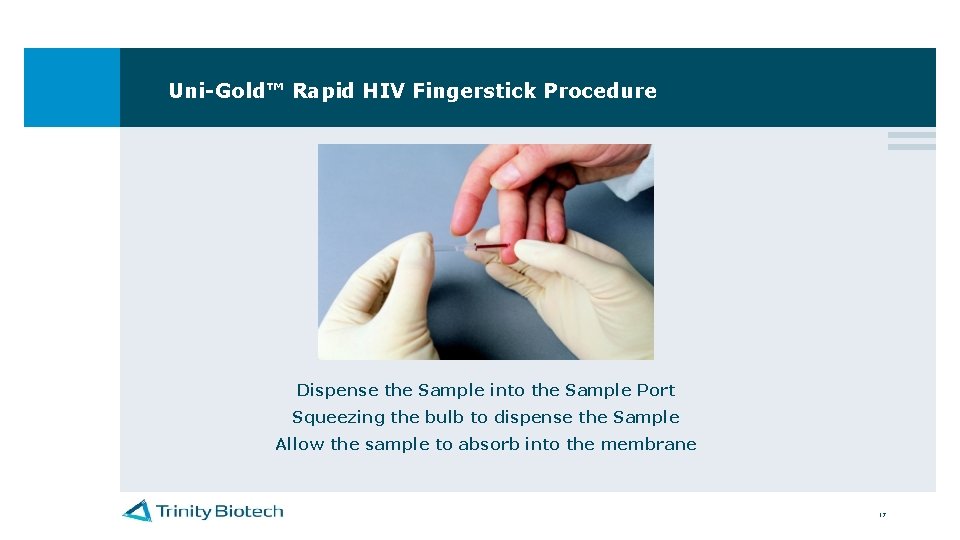
Uni-Gold™ Rapid HIV Fingerstick Procedure Dispense the Sample into the Sample Port Squeezing the bulb to dispense the Sample Allow the sample to absorb into the membrane 17

Uni-Gold™ Rapid HIV Fingerstick Procedure Add 4 Drops of Wash Solution into the Sample Port Do not touch the sample port with the tip of the wash solution 18
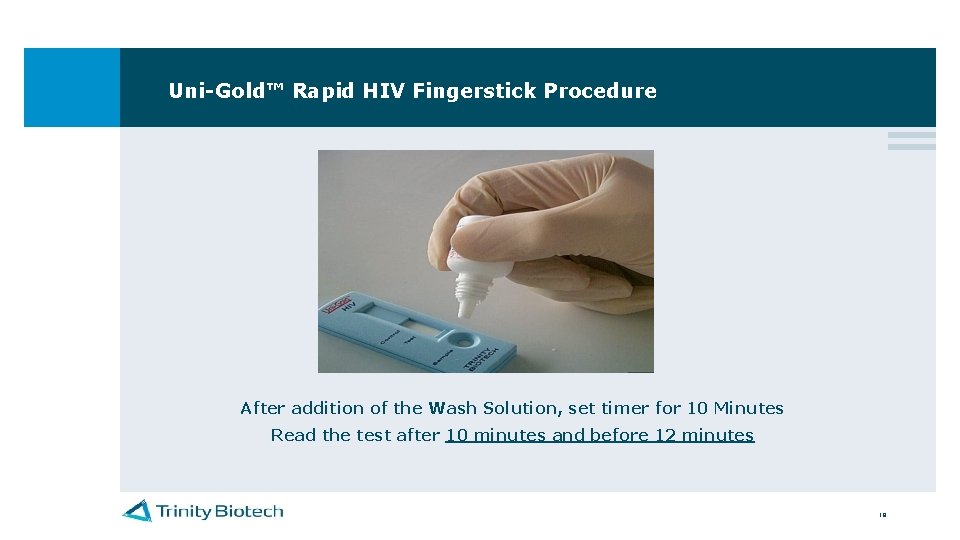
Uni-Gold™ Rapid HIV Fingerstick Procedure After addition of the Wash Solution, set timer for 10 Minutes Read the test after 10 minutes and before 12 minutes 19

Uni-Gold™ Rapid HIV Fingerstick Procedure For a CLIA waived test result to be valid, there must be a control line present and full red color in the sample port Control Line Area Test Line Area Sample Port 20
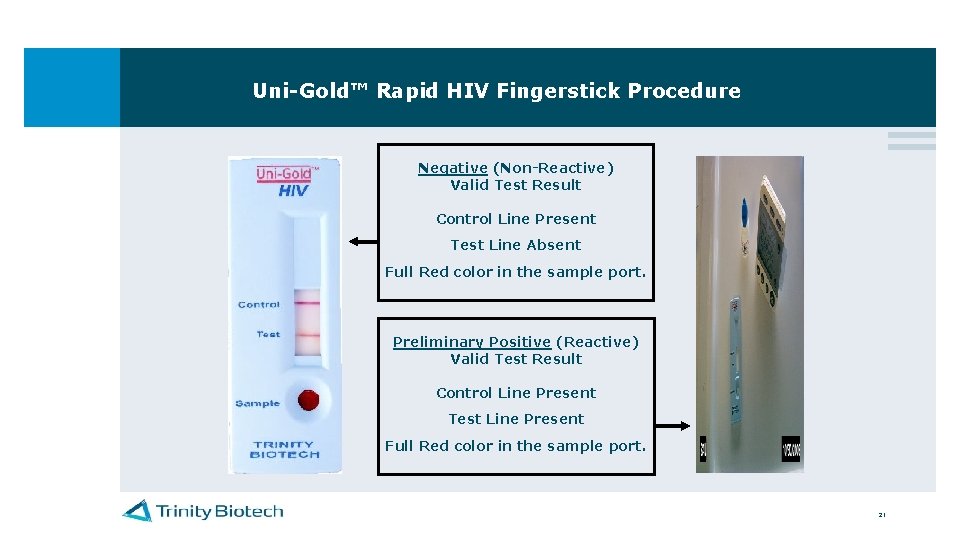
Uni-Gold™ Rapid HIV Fingerstick Procedure Negative (Non-Reactive) Valid Test Result Control Line Present Test Line Absent Full Red color in the sample port. Preliminary Positive (Reactive) Valid Test Result Control Line Present Test Line Present Full Red color in the sample port. 21

Uni-Gold™ Quality Control (QC) Performance “When do I need to run controls? ” – – – Have I ever ran External Controls in the past? Has Anyone ran External Controls with this Lot Number? Has Anyone ran External Controls with this Shipment? Have the storage temperatures been acceptable? Am I in compliance with our Quality Assurance Guidelines? If the answer to any of the above is “No”, you will need to perform the external QC. 22
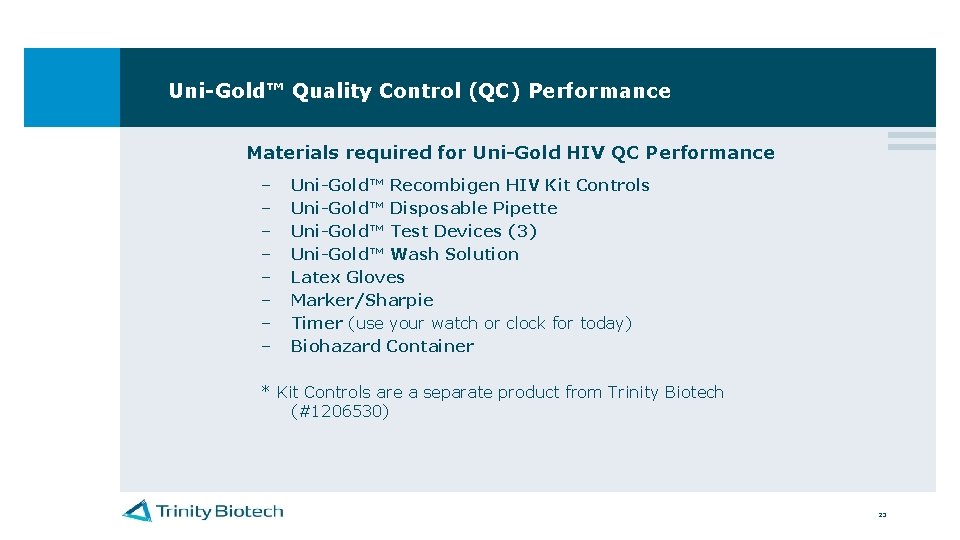
Uni-Gold™ Quality Control (QC) Performance Materials required for Uni-Gold HIV QC Performance – – – – Uni-Gold™ Recombigen HIV Kit Controls Uni-Gold™ Disposable Pipette Uni-Gold™ Test Devices (3) Uni-Gold™ Wash Solution Latex Gloves Marker/Sharpie Timer (use your watch or clock for today) Biohazard Container * Kit Controls are a separate product from Trinity Biotech (#1206530) 23

Uni-Gold™ Quality Control (QC) Performance Organize all materials required for External QC Performance Open new Test Devices (3) and Mark with QC ID (1+, 2+, Neg) If the QC Vials are unopened, record date on vial/box 24

Uni-Gold™ Quality Control (QC) Performance Immerse the tip of the Disposable Pipette into the QC Vial GENTLY press on the bulb to draw a minimal amount of sample From vertical, dispense 1 free-falling Drop into the sample port Allow to absorb into the sample port 25
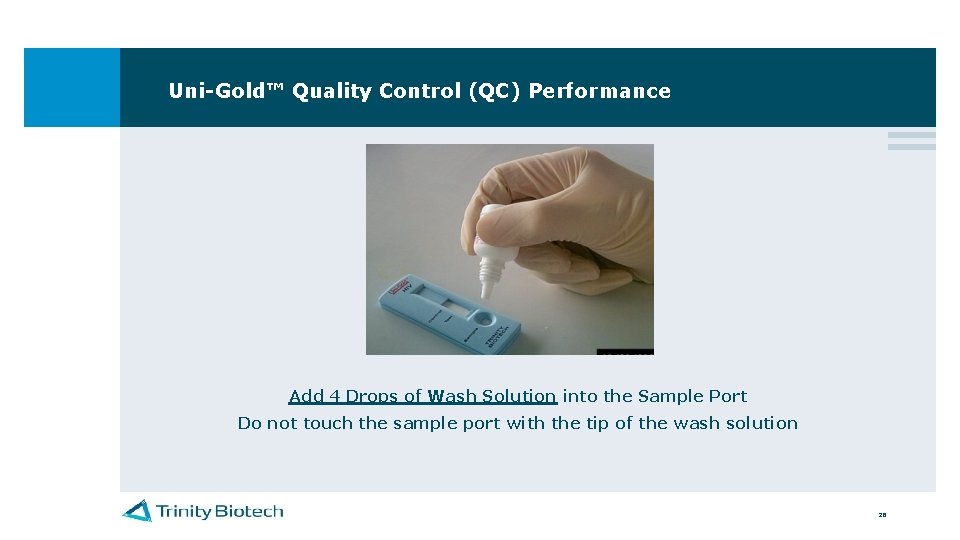
Uni-Gold™ Quality Control (QC) Performance Add 4 Drops of Wash Solution into the Sample Port Do not touch the sample port with the tip of the wash solution 26

Uni-Gold™ Quality Control (QC) Performance After addition of the wash solution, set timer for 10 Minutes Read test after 10 minutes and before 12 minutes 27
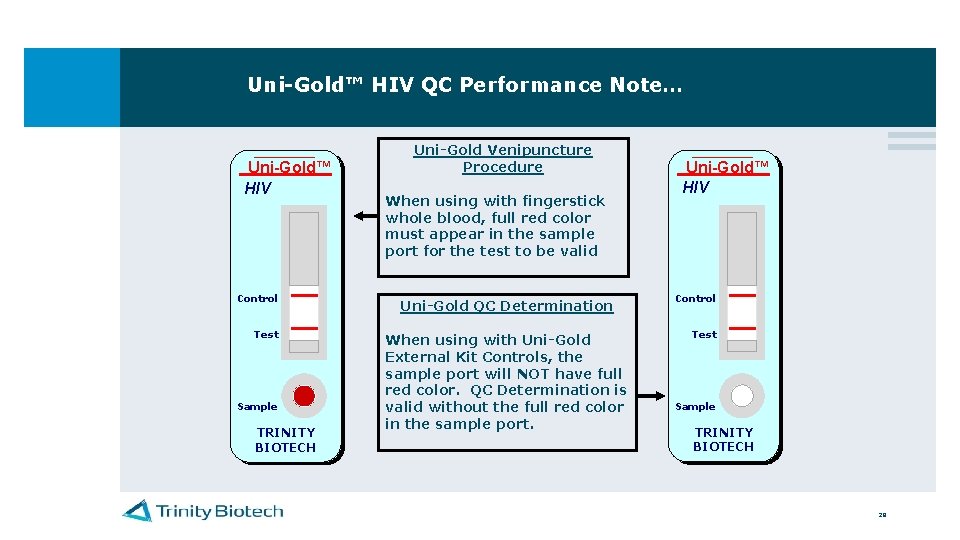
Uni-Gold™ HIV QC Performance Note… Uni-Gold™ HIV Control Test Sample TRINITY BIOTECH Uni-Gold Venipuncture Procedure When using with fingerstick whole blood, full red color must appear in the sample port for the test to be valid Uni-Gold QC Determination When using with Uni-Gold External Kit Controls, the sample port will NOT have full red color. QC Determination is valid without the full red color in the sample port. Uni-Gold™ HIV Control Test Sample TRINITY BIOTECH 28

• The link below is to an video demonstration of the Uni. Gold Recombigen HIV 1/2 Fingerstick Procedure. Please note this is for use as a training guide only. The most current Instructions should always be reviewed prior to testing. ” • https: //youtu. be/w. WSn. UWfr. H 2 k

Thank you for participating in the Unigold Recombigen HIV 1/2 Training for HIV Testing Sites. Please direct any of your questions or concerns to your Regional Coordinator.

CLINICAL CHEMISTRY | COAGULATION | INFECTIOUS DISEASE | POINT OF CARE Uni-Gold™ Recombigen HIV 1/2 Training Lou Pastors MT(ASCP National Account Manager Trinity Biotech Phone: 630 -561 -6681 e. Mail: louis. pastors@trinityusa. com 31
- Slides: 31