UNIFR Sandro Rusconi 2003 1972 75 1975 79
















- Slides: 48

UNIFR Sandro Rusconi 2003 1972 -75 1975 -79 1979 -82 1982 -84 1984 -86 1987 -91 1994 -today 1995 -today 2002 -03 2002 -05 School teacher (Locarno, Switzerland) Graduation in Biology UNI Zuerich, Switzerland Ph. D curriculum UNI Zuerich, molecular biology Research assistant UNI Zuerich Postdoc UCSF, K Yamamoto, (San Francisco) Principal Investigator, UNI Zuerich Professor Biochemistry UNI Fribourg Director Swiss National Research Program 37 'Somatic Gene Therapy' Sabbatical, Tufts Med. School Boston and Univ. Milano, Pharmacology Department President Union of Swiss Societies for Experimental Biology (USGEB) Feb 19, 2003 ECPM Basel 2003: Gene therapy turning teenage, what have we learned?

Genetics has been used since millennia, Molecular Biology, only since 30 years 100’ 000 b. C. Empirical genetics 10’ 000 b. C. Biotechnology 2000 a. d. Molecular biology 2001 a. d, Genomics UNIFR Rusconi 2003

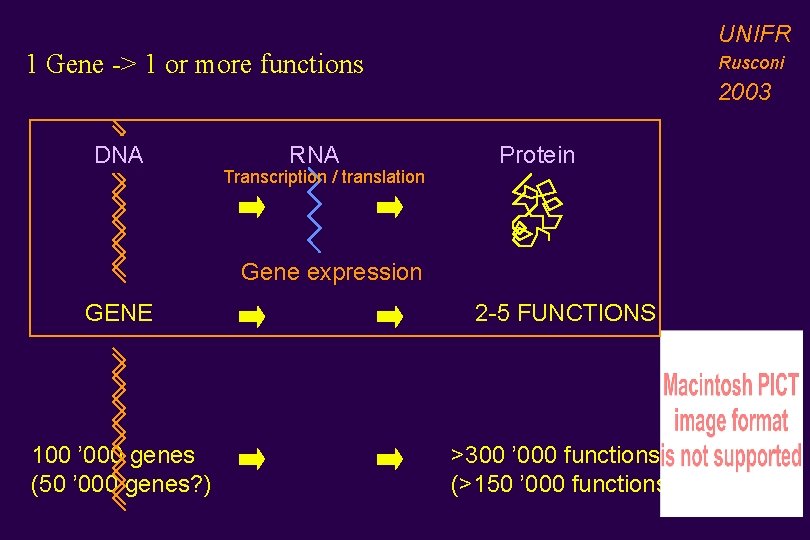
UNIFR 1 Gene -> 1 or more functions DNA RNA Transcription / translation Rusconi 2003 Protein Gene expression GENE 2 -5 FUNCTIONS 100 ’ 000 genes (50 ’ 000 genes? ) >300 ’ 000 functions (>150 ’ 000 functions)

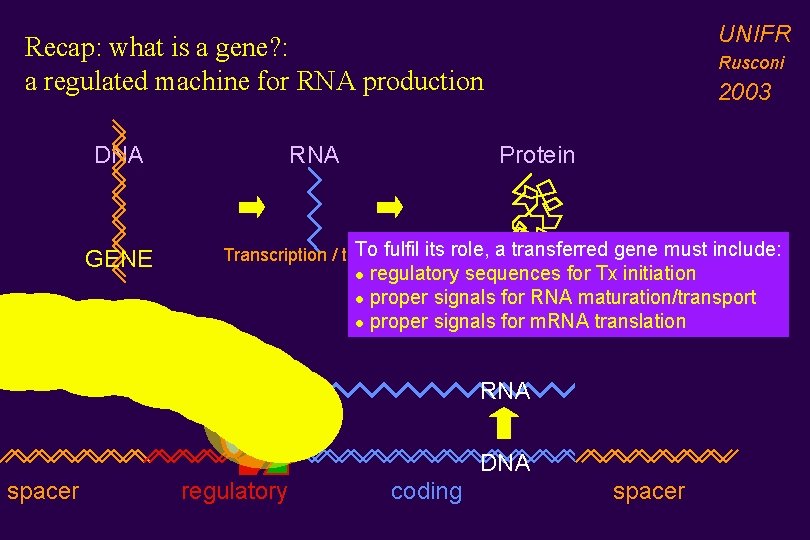
UNIFR Recap: what is a gene? : a regulated machine for RNA production DNA GENE RNA Rusconi 2003 Protein To fulfil its role, a transferred gene must include: Transcription / translation FUNCTION regulatory sequences for Tx initiation l proper signals for RNA maturation/transport l proper signals for m. RNA translation l RNA DNA spacer regulatory coding spacer

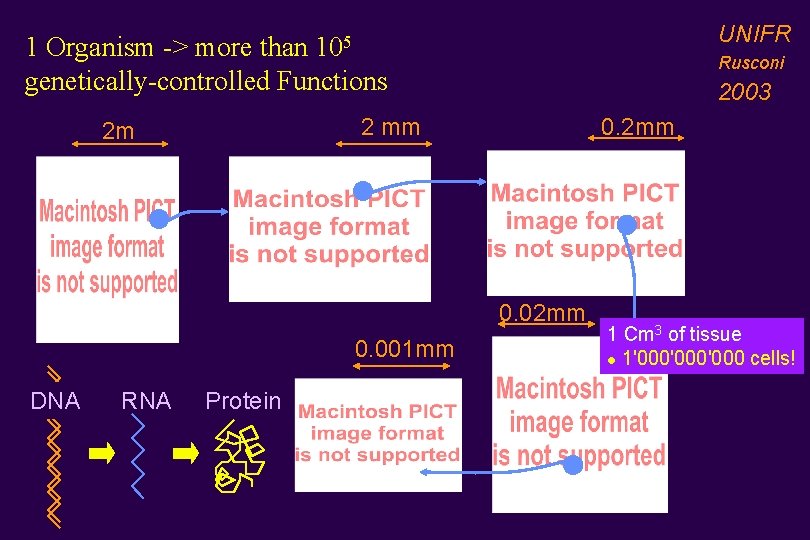
UNIFR 1 Organism -> more than 105 genetically-controlled Functions Rusconi 2003 2 mm 2 m 0. 2 mm 0. 001 mm DNA RNA Protein 1 Cm 3 of tissue l 1'000'000 cells!

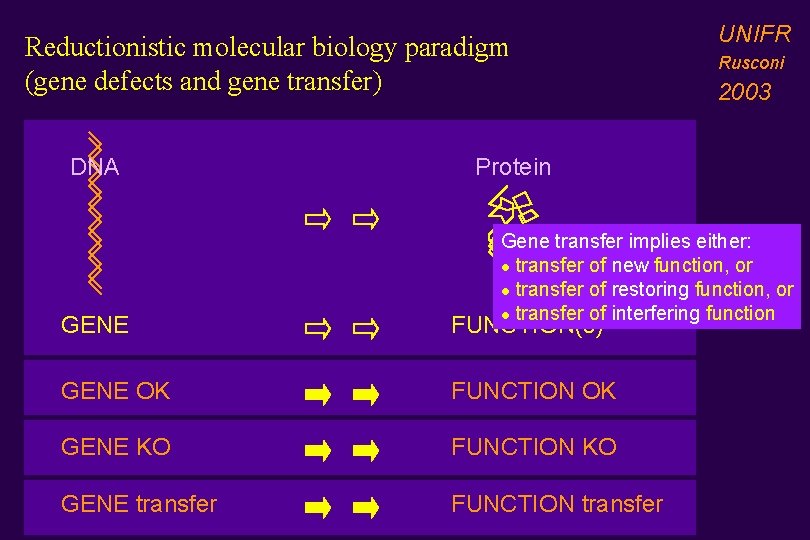
Reductionistic molecular biology paradigm (gene defects and gene transfer) DNA UNIFR Rusconi 2003 Protein Gene transfer implies either: l transfer of new function, or l transfer of restoring function, or l transfer of interfering function GENE FUNCTION(s) GENE OK FUNCTION OK GENE KO FUNCTION KO GENE transfer FUNCTION transfer

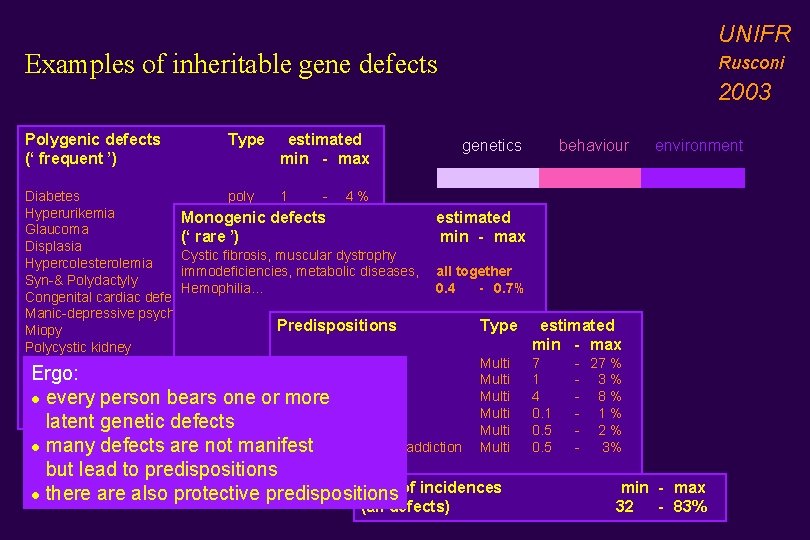
UNIFR Examples of inheritable gene defects Polygenic defects (‘ frequent ’) Type estimated min - max Rusconi 2003 genetics Diabetes poly 1 - 4 % Hyperurikemia Multi 2 - 15 % Monogenic defects estimated Glaucoma poly 1 - 2 % (‘ rare ’) min - max Displasia Multi 1 - 3 % Cystic fibrosis, muscular dystrophy Hypercolesterolemia Multi 1 - 5 % immodeficiencies, metabolic diseases, all together Syn-& Polydactyly poly 0. 1 - 1 % Hemophilia. . . 0. 4 - 0. 7% Congenital cardiac defects Multi 0. 5 - 0. 8 % Manic-depressive psychosis Multi 0. 4 - 3 % Predispositions Type Miopy poly 3 - 4 % Polycystic kidney poly 0. 1 - 1 % Multi Psoriasis Multi (*) Alzheimer 2 - 3 % Ergo: Multi Schizofrenia Multi (*) Parkinson 0. 5 - 1 % Multi Scoliosis Multi (*) Breast cancer 3 - 5 % l every person bears one or more (*) Colon Carcinoma Multi latent genetic defects (*) Obesity Multi (*) Alcolholism/ drug addiction Multi l many defects are not manifest but lead to predispositions Sum of incidences l there also protective predispositions (all defects) behaviour environment estimated min - max 7 1 4 0. 1 0. 5 - 27 % - 3 % - 8 % - 1 % - 2 % 3% min - max 32 - 83%

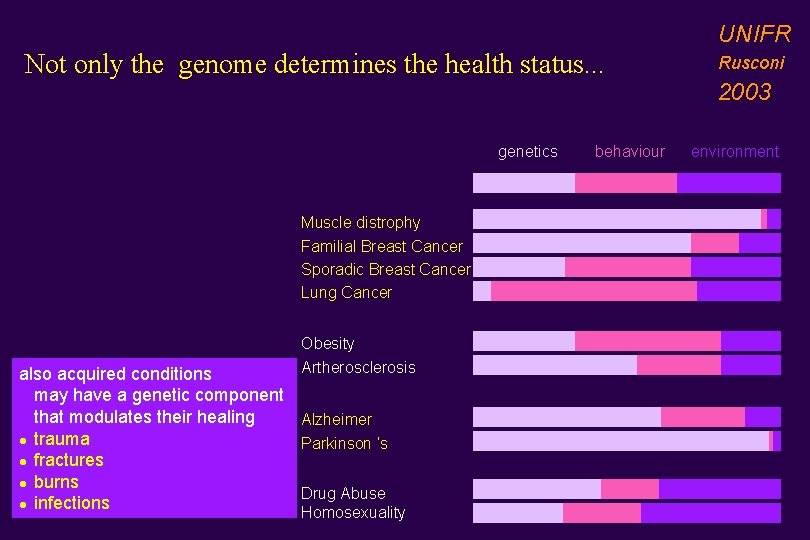
UNIFR Not only the genome determines the health status. . . genetics Muscle distrophy Familial Breast Cancer Sporadic Breast Cancer Lung Cancer also acquired conditions may have a genetic component that modulates their healing l trauma l fractures l burns l infections Obesity Artherosclerosis Alzheimer Parkinson ’s Drug Abuse Homosexuality behaviour Rusconi 2003 environment

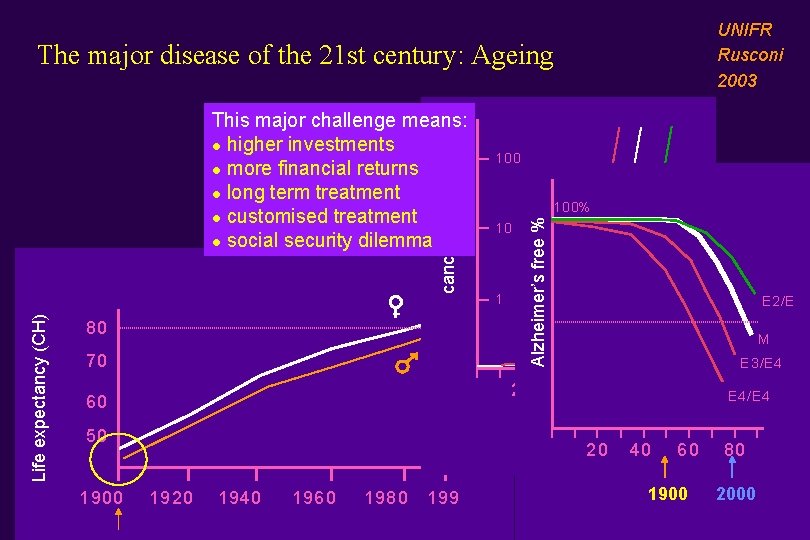
UNIFR Rusconi 2003 The major disease of the 21 st century: Ageing 80 70 100% 10 1 20 60 E 2/E 4 40 60 80 1900 20 40 60 50 1900 100 Alzheimer’s free % Life expectancy (CH) cancer incidence This major challenge means: l higher investments l more financial returns l long term treatment l customised treatment l social security dilemma 1920 1940 1960 1980 199 1900 M E 3/E 4 E 4/E 4 80 2000

UNIFR Rusconi 2003 The THREE missions of medicine Prevention + 'Molecular Medicine' Diagnosis Application of the know-how in molecular genetics to medicine + + Therapy

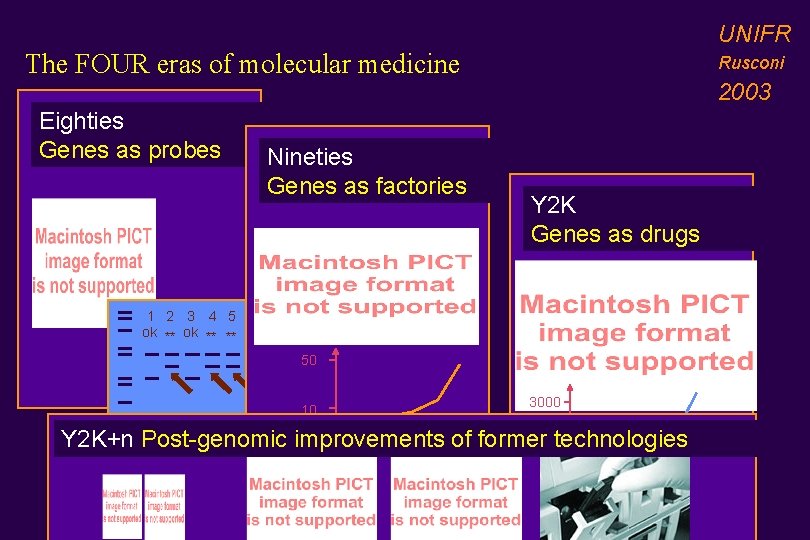
UNIFR The FOUR eras of molecular medicine Eighties Genes as probes Nineties Genes as factories Rusconi 2003 Y 2 K Genes as drugs 1 2 3 4 5 ok ** ** 50 10 3000 80 85 90 95 99 1000 Y 2 K+n Post-genomic improvements of former technologies 80 85 90 95 00

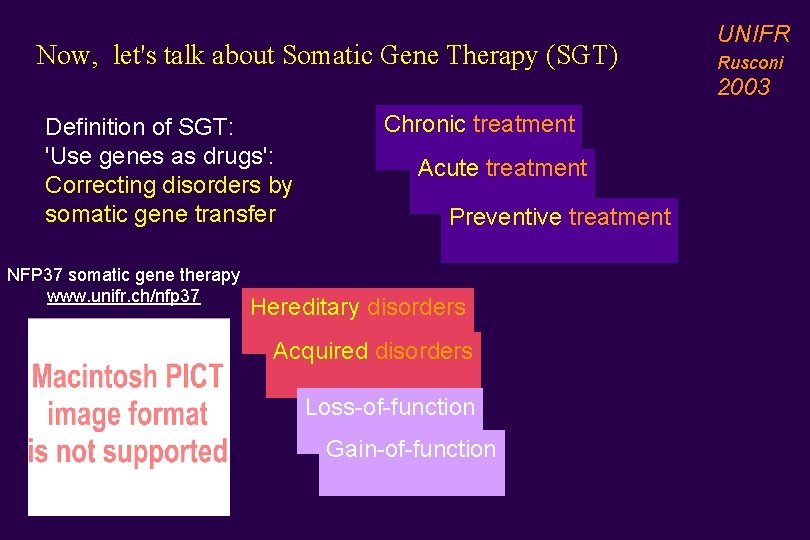
Now, let's talk about Somatic Gene Therapy (SGT) UNIFR Rusconi 2003 Definition of SGT: 'Use genes as drugs': Correcting disorders by somatic gene transfer NFP 37 somatic gene therapy www. unifr. ch/nfp 37 Chronic treatment Acute treatment Preventive treatment Hereditary disorders Acquired disorders Loss-of-function Gain-of-function

The SGTtherapy principle is teenage simple Yes, . . . Gene turns in 2003, but: buthas theitdevil often in the details reallyisgrown up? UNIFR Rusconi 2003 There are many things that are simple in principle, like. . . First clinical trial of a monogenic disease 1990 F. Anderson & Co: ADA deficiency getting a train ticket. . . does not work ! try this 5 min before departure and with a group of Chinese tourists in front parking your car. . . 2002 ! try this at noon, any given day Same protocol as Anderson's for ADA in Zuerich or Geneva. . . gene therapy (C. Bordignon) counting votes. . . it works! ! ask Florida's officials. . . gene therapy. . . look at progress in 13 years. . .

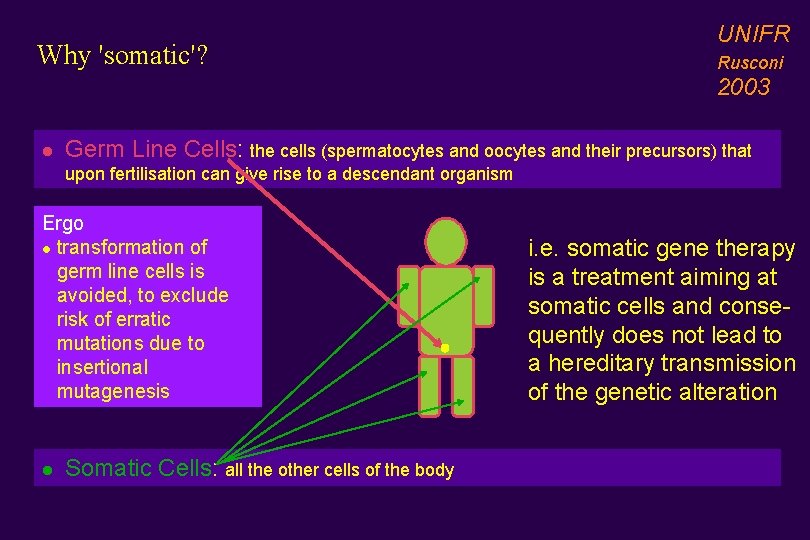
Why 'somatic'? UNIFR Rusconi 2003 l Germ Line Cells: the cells (spermatocytes and oocytes and their precursors) that upon fertilisation can give rise to a descendant organism Ergo l transformation of germ line cells is avoided, to exclude risk of erratic mutations due to insertional mutagenesis l Somatic Cells: all the other cells of the body i. e. somatic gene therapy is a treatment aiming at somatic cells and consequently does not lead to a hereditary transmission of the genetic alteration

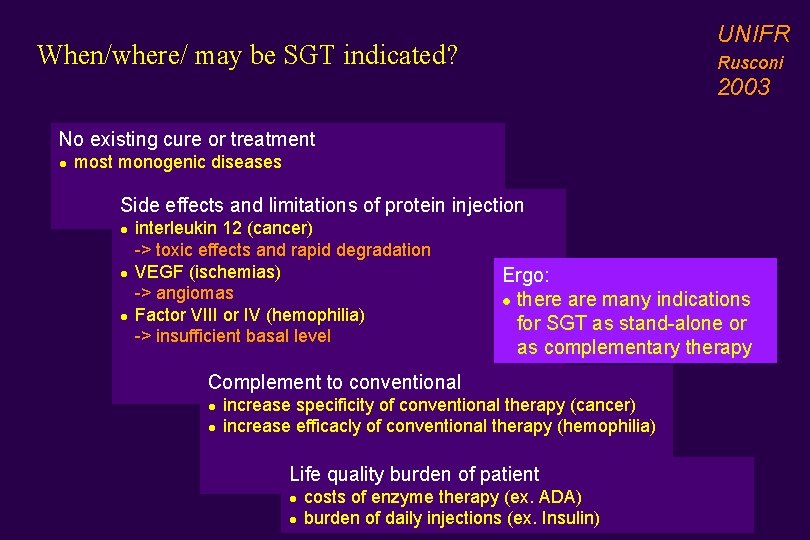
UNIFR When/where/ may be SGT indicated? Rusconi 2003 No existing cure or treatment l most monogenic diseases Side effects and limitations of protein injection l l l interleukin 12 (cancer) -> toxic effects and rapid degradation VEGF (ischemias) -> angiomas Factor VIII or IV (hemophilia) -> insufficient basal level Ergo: l there are many indications for SGT as stand-alone or as complementary therapy Complement to conventional l l increase specificity of conventional therapy (cancer) increase efficacly of conventional therapy (hemophilia) Life quality burden of patient l l costs of enzyme therapy (ex. ADA) burden of daily injections (ex. Insulin)

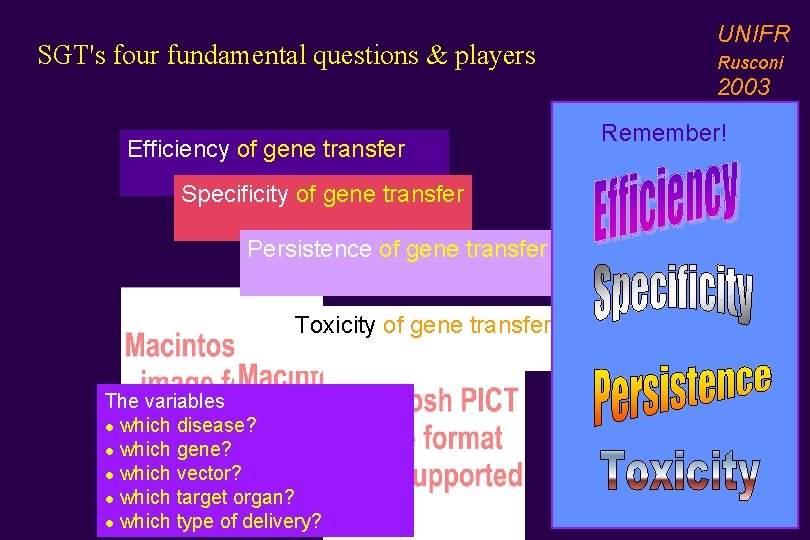
SGT's four fundamental questions & players UNIFR Rusconi 2003 Efficiency of gene transfer Specificity of gene transfer Persistence of gene transfer Toxicity of gene transfer The variables l which disease? l which gene? l which vector? l which target organ? l which type of delivery? Remember!

The SGT acrobatics: matching vectors / delivery system / disease Chronic Conditions l l l Slow onset of expression acceptable Initiation of the treatment weeks/months/years before 'point of no return' (ex. cystic fibrosis) persisting expression of the transgene or re-administration required (example hemophilia) Usually based on compensation of 'genetic loss-of-function' (permanent re-gain of function; ex. ADA) Regulation of gene expression often necessary (because of persistence) For some diseases even a small % of tissue transformation is already therapeutic UNIFR Rusconi 2003 Acute Conditions l l l Rapid onset of expression necessary Initiation of the treatment minutes/hours/days before 'point of no return' (ex. brain ischemia) persisting expression of the transgene not required, occasional re-administration (example Usually based on augmentation of resident function (transient gain of function; ex. VEGF) Regulation of gene expression not necessary (because of transiency) For most diseases even a small % of transformation is already therapeutic Ergo l many divergent variables must be matched for each case l an advantage for one purpose becomes a disadvantage for another (viceversa)

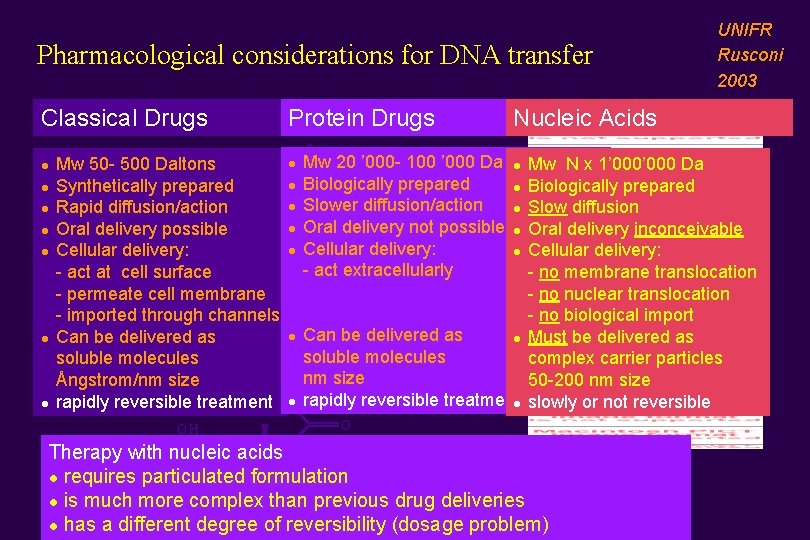
Pharmacological considerations for DNA transfer Classical Drugs l l l l Mw 50 - 500 Daltons OH Synthetically prepared Rapid diffusion/action Oral delivery possible Cellular delivery: O - act at cell surface - permeate cell membrane OH - imported through channels Can be delivered as soluble molecules Ångstrom/nm size rapidly reversible treatment O OH Protein Drugs l l l l Mw 20 ’ 000 - 100 ’ 000 Da OH Biologically prepared Slower diffusion/action Oral delivery not possible Cellular delivery: - act extracellularly UNIFR Rusconi 2003 Nucleic Acids Mw N x 1’ 000 Da l Biologically prepared l Slow diffusion l Oral delivery inconceivable l Cellular delivery: - no membrane translocation - no nuclear translocation O - no biological import OH Can be delivered as l Must be delivered as soluble molecules complex carrier particles nm size 50 -200 nm size rapidly reversible treatmentl slowly or not reversible O OH l Therapy with nucleic acids l requires particulated formulation l is much more complex than previous drug deliveries l has a different degree of reversibility (dosage problem) O

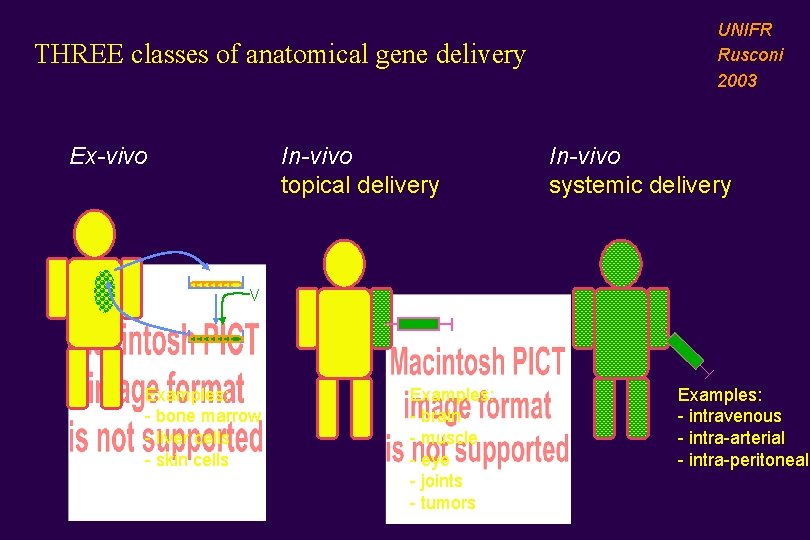
THREE classes of anatomical gene delivery Ex-vivo In-vivo topical delivery UNIFR Rusconi 2003 In-vivo systemic delivery V Examples: - bone marrow - liver cells - skin cells Examples: - brain - muscle - eye - joints - tumors Examples: - intravenous - intra-arterial - intra-peritoneal

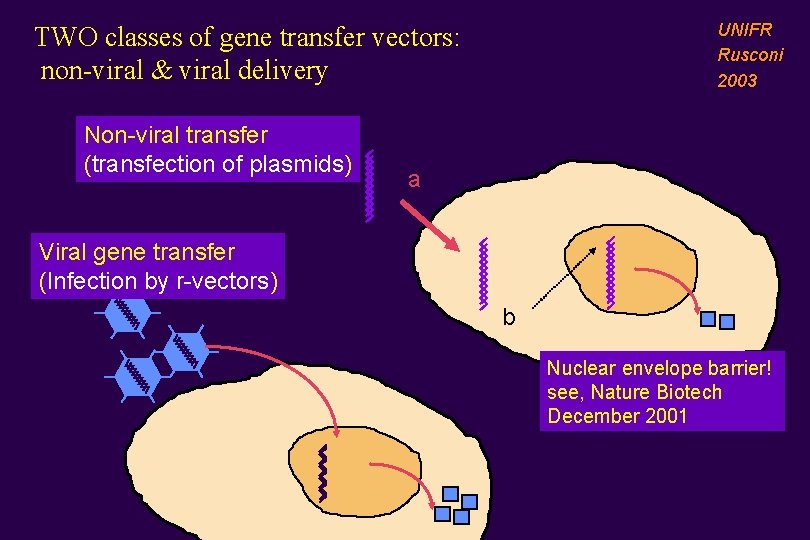
UNIFR Rusconi 2003 TWO classes of gene transfer vectors: non-viral & viral delivery Non-viral transfer (transfection of plasmids) a Viral gene transfer (Infection by r-vectors) b Nuclear envelope barrier! see, Nature Biotech December 2001

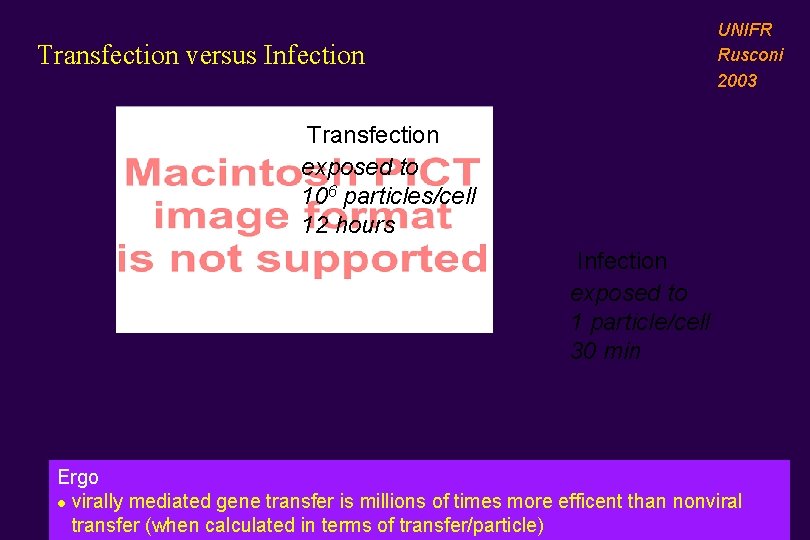
UNIFR Rusconi 2003 Transfection versus Infection Transfection exposed to 106 particles/cell 12 hours Infection exposed to 1 particle/cell 30 min Ergo l virally mediated gene transfer is millions of times more efficent than nonviral transfer (when calculated in terms of transfer/particle)

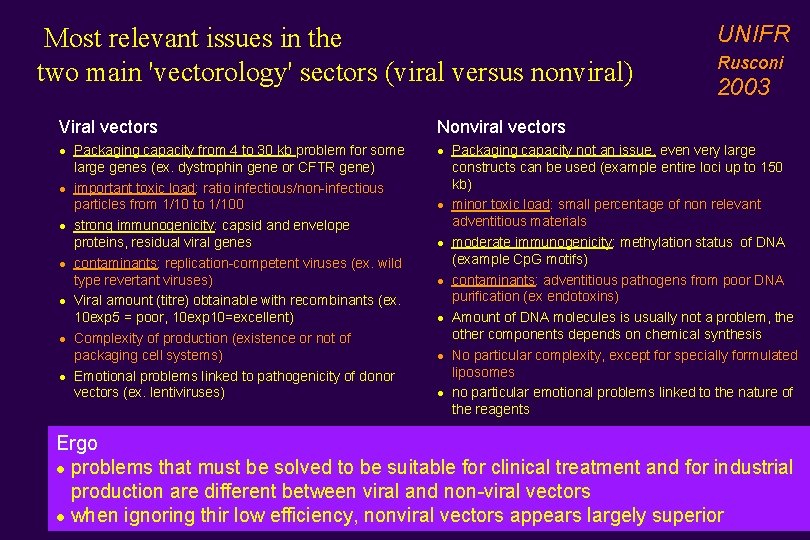
Most relevant issues in the two main 'vectorology' sectors (viral versus nonviral) Viral vectors l l l l Packaging capacity from 4 to 30 kb problem for some large genes (ex. dystrophin gene or CFTR gene) important toxic load: ratio infectious/non-infectious particles from 1/10 to 1/100 strong immunogenicity: capsid and envelope proteins, residual viral genes contaminants: replication-competent viruses (ex. wild type revertant viruses) Viral amount (titre) obtainable with recombinants (ex. 10 exp 5 = poor, 10 exp 10=excellent) Complexity of production (existence or not of packaging cell systems) Emotional problems linked to pathogenicity of donor vectors (ex. lentiviruses) UNIFR Rusconi 2003 Nonviral vectors l l l l Packaging capacity not an issue, even very large constructs can be used (example entire loci up to 150 kb) minor toxic load: small percentage of non relevant adventitious materials moderate immunogenicity: methylation status of DNA (example Cp. G motifs) contaminants: adventitious pathogens from poor DNA purification (ex endotoxins) Amount of DNA molecules is usually not a problem, the other components depends on chemical synthesis No particular complexity, except for specially formulated liposomes no particular emotional problems linked to the nature of the reagents Ergo l problems that must be solved to be suitable for clinical treatment and for industrial production are different between viral and non-viral vectors l when ignoring thir low efficiency, nonviral vectors appears largely superior

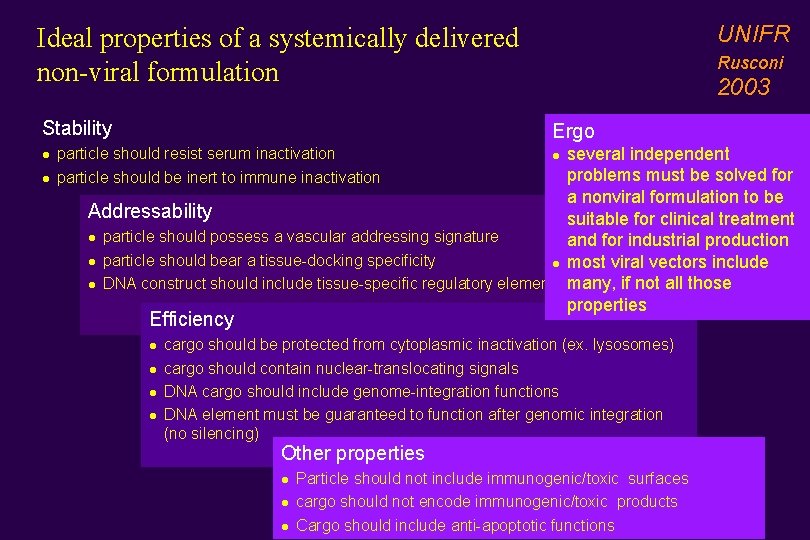
UNIFR Ideal properties of a systemically delivered non-viral formulation Stability l l Rusconi 2003 Ergo several independent problems must be solved for a nonviral formulation to be Addressability suitable for clinical treatment l particle should possess a vascular addressing signature and for industrial production l particle should bear a tissue-docking specificity l most viral vectors include l DNA construct should include tissue-specific regulatory elements many, if not all those properties particle should resist serum inactivation particle should be inert to immune inactivation l Efficiency l l cargo should be protected from cytoplasmic inactivation (ex. lysosomes) cargo should contain nuclear-translocating signals DNA cargo should include genome-integration functions DNA element must be guaranteed to function after genomic integration (no silencing) Other properties l l l Particle should not include immunogenic/toxic surfaces cargo should not encode immunogenic/toxic products Cargo should include anti-apoptotic functions

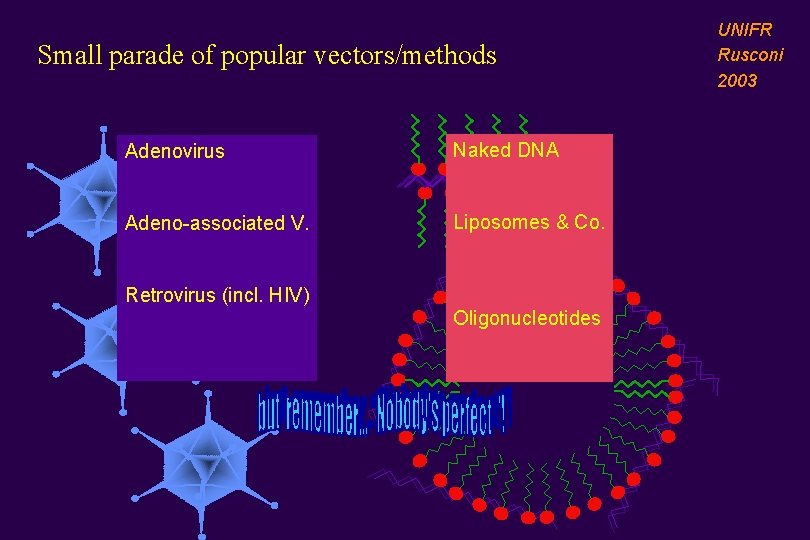
Small parade of popular vectors/methods Adenovirus Naked DNA Adeno-associated V. Liposomes & Co. Retrovirus (incl. HIV) Oligonucleotides UNIFR Rusconi 2003

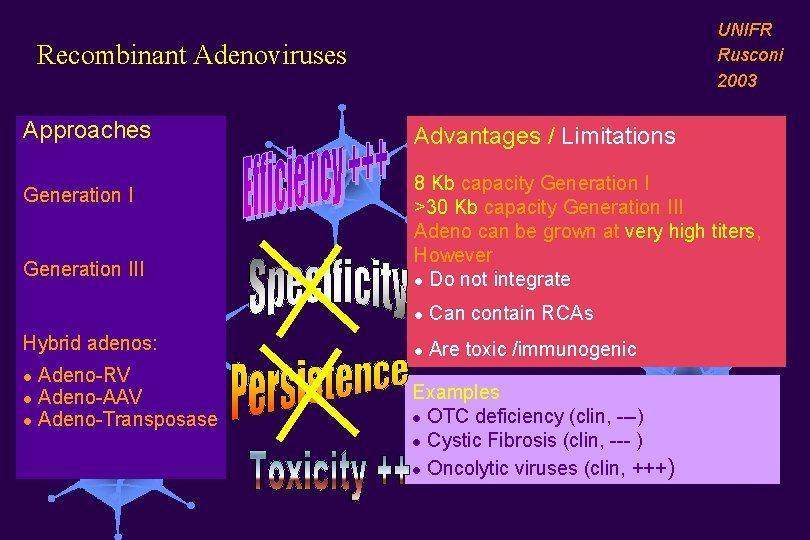
UNIFR Rusconi 2003 Recombinant Adenoviruses Approaches Generation III Hybrid adenos: Adeno-RV l Adeno-AAV l Adeno-Transposase l Advantages / Limitations 8 Kb capacity Generation I >30 Kb capacity Generation III Adeno can be grown at very high titers, However l Do not integrate l Can contain RCAs l Are toxic /immunogenic Examples l OTC deficiency (clin, ---) l Cystic Fibrosis (clin, --- ) l Oncolytic viruses (clin, +++)

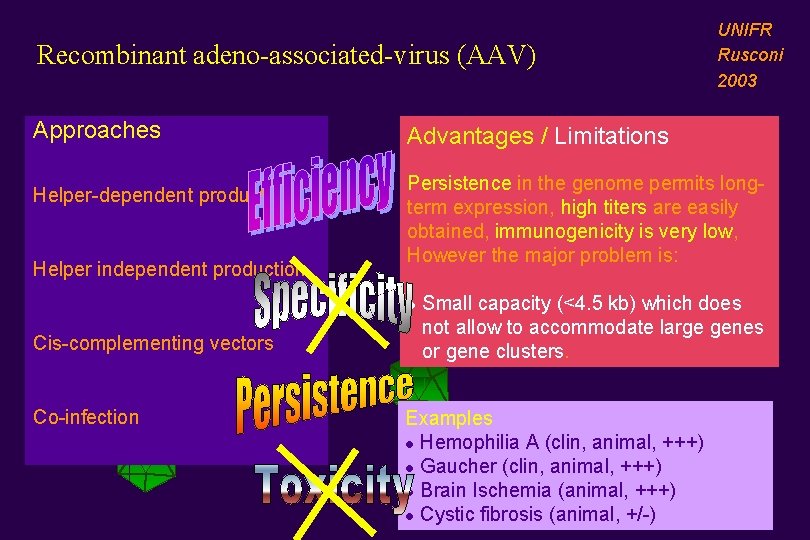
Recombinant adeno-associated-virus (AAV) Approaches Helper-dependent production Helper independent production Advantages / Limitations Persistence in the genome permits longterm expression, high titers are easily obtained, immunogenicity is very low, However the major problem is: l Cis-complementing vectors Co-infection UNIFR Rusconi 2003 Small capacity (<4. 5 kb) which does not allow to accommodate large genes or gene clusters. Examples l Hemophilia A (clin, animal, +++) l Gaucher (clin, animal, +++) l Brain Ischemia (animal, +++) l Cystic fibrosis (animal, +/-)

Recombinant Retroviruses (includes HIV-based) Approaches Murine Retroviruses VSV-pseudotyped RV Lentiviruses ! Self-inactivating RV Combination viruses UUNIFR Rusconi 2003 Advantages / Limitations 9 Kb capacity + integration through transposition also in quiescent cells (HIV), permit in principle long-term treatments, however disturbed by: l Insertional mutagenesis l Gene silencing l High mutation rate l Low titer of production Examples l SCID (IL 2 R defect, Paris) (clin, +++) l Adenosine Deaminase deficiency (clin, +++!!!) l Parkinson (preclin, +++) l Anti cancer (clin +/-)

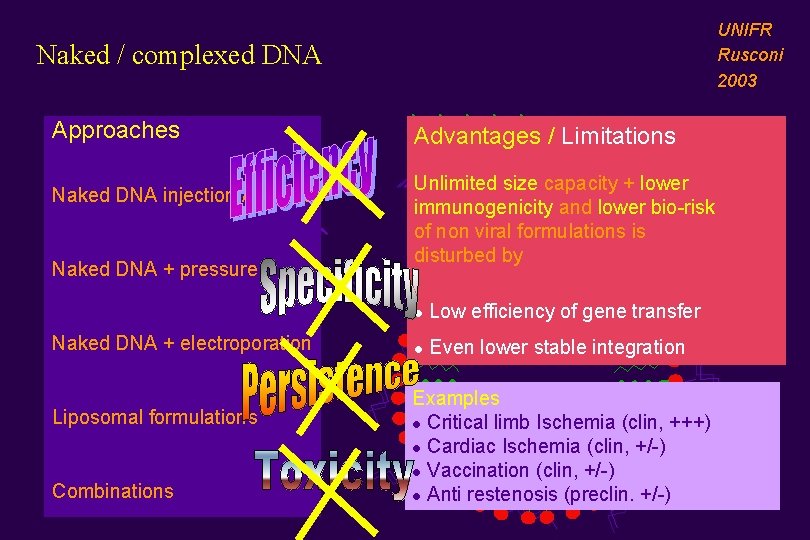
UNIFR Rusconi 2003 Naked / complexed DNA Approaches Naked DNA injection /biolistic Naked DNA + pressure Naked DNA + electroporation Liposomal formulations Combinations Advantages / Limitations Unlimited size capacity + lower immunogenicity and lower bio-risk of non viral formulations is disturbed by l Low efficiency of gene transfer l Even lower stable integration Examples l Critical limb Ischemia (clin, +++) l Cardiac Ischemia (clin, +/-) l Vaccination (clin, +/-) l Anti restenosis (preclin. +/-)

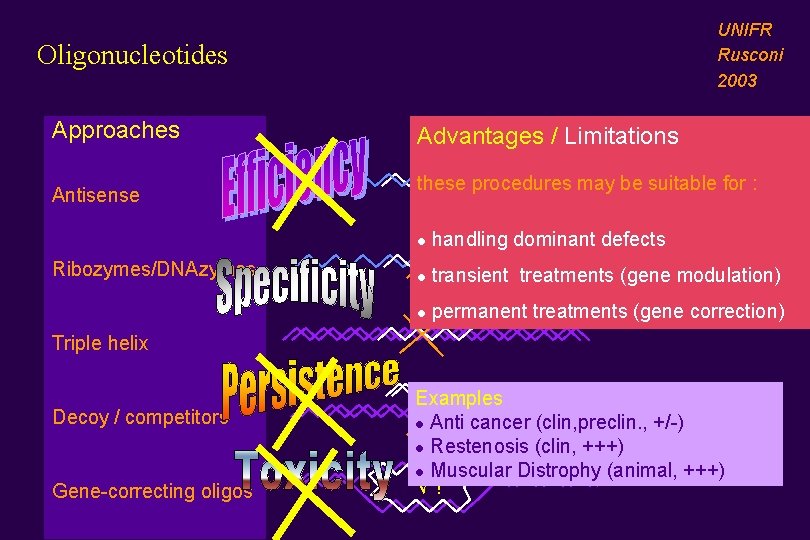
UNIFR Rusconi 2003 Oligonucleotides Approaches Antisense Ribozymes/DNAzymes Advantages / Limitations these procedures may be suitable for : l handling dominant defects l transient treatments (gene modulation) l permanent treatments (gene correction) Triple helix Decoy / competitors Gene-correcting oligos Examples l Anti cancer (clin, preclin. , +/-) l Restenosis (clin, +++) l Muscular Distrophy (animal, +++) √ !

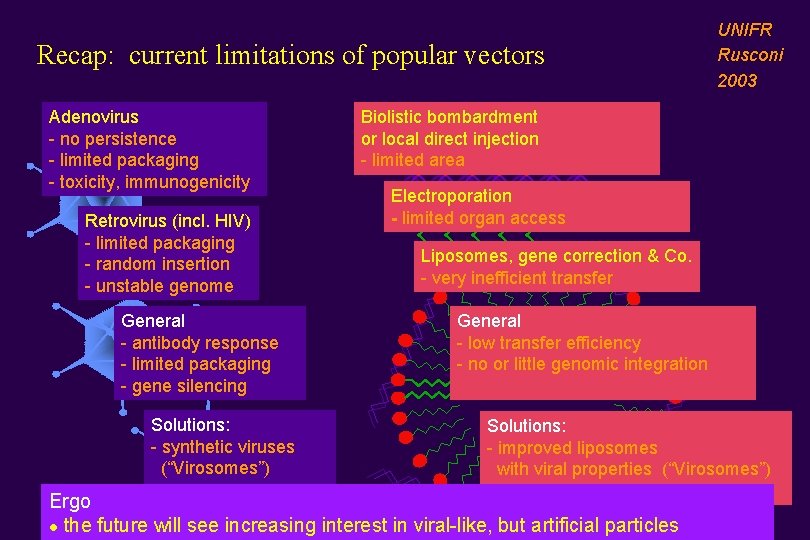
Recap: current limitations of popular vectors Adenovirus - no persistence - limited packaging - toxicity, immunogenicity Retrovirus (incl. HIV) - limited packaging - random insertion - unstable genome General - antibody response - limited packaging - gene silencing Solutions: - synthetic viruses (“Virosomes”) UNIFR Rusconi 2003 Biolistic bombardment or local direct injection - limited area Electroporation - limited organ access Liposomes, gene correction & Co. - very inefficient transfer General - low transfer efficiency - no or little genomic integration Solutions: - improved liposomes with viral properties (“Virosomes”) Ergo l the future will see increasing interest in viral-like, but artificial particles

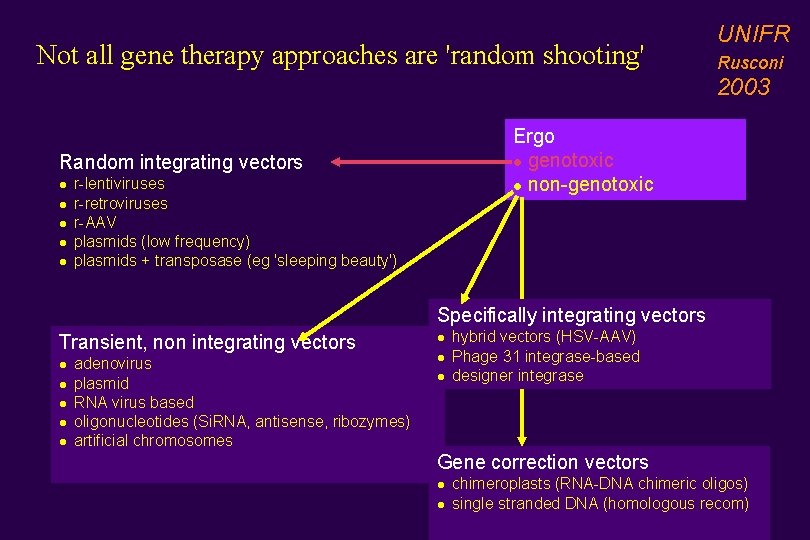
Not all gene therapy approaches are 'random shooting' UNIFR Rusconi 2003 Ergo l genotoxic l non-genotoxic Random integrating vectors l l l r-lentiviruses r-retroviruses r-AAV plasmids (low frequency) plasmids + transposase (eg 'sleeping beauty') Specifically integrating vectors Transient, non integrating vectors l l l adenovirus plasmid RNA virus based oligonucleotides (Si. RNA, antisense, ribozymes) artificial chromosomes l l l hybrid vectors (HSV-AAV) Phage 31 integrase-based designer integrase Gene correction vectors l l chimeroplasts (RNA-DNA chimeric oligos) single stranded DNA (homologous recom)

Which vector for which disease category UNIFR Rusconi 2003 Disease Type Most suitable vector Justifications /Issues Chronic Metabolic AAV, Lenti, Adeno III, rretroviruses, repair oligo persistence of expression of the transferred gene, minimize readministration AAV, nonviral, Lenti No rapid expression necessary, persistence required, low toxicity Adeno II, Plasmid, oncolytic recombinant viruses rapid & transient expression of cytotoxic or immunomodulators Adeno II, Plasmid, modulatory oligonucleotides Rapid and transient action required (ex. OTC, Gaucher, Haemophilia, hematopoietic) Local chronic or progressive (ex. CNS, joints, eyes) Solid tumors +/- metastat. (cervical, breast, brain, skin) Trauma or infection (Ischemia, fracture, burn, wound, acute infection, anaphyllaxis)

Technologies related to-, but not genuinely definable as 'gene therapy' Bioactive oligonucleotides antisense l decoy ds. DNA l decoy RNA l ribozymes DNAzymes l Si RNA l Oncolytic viruses ONYX-15, ONYX-638 (r-adeno) l r-HSV l r-FSV l Implants of encapsulated cells neurotrophic factor producer cell implants l hormone-producing cells l UNIFR Rusconi 2003

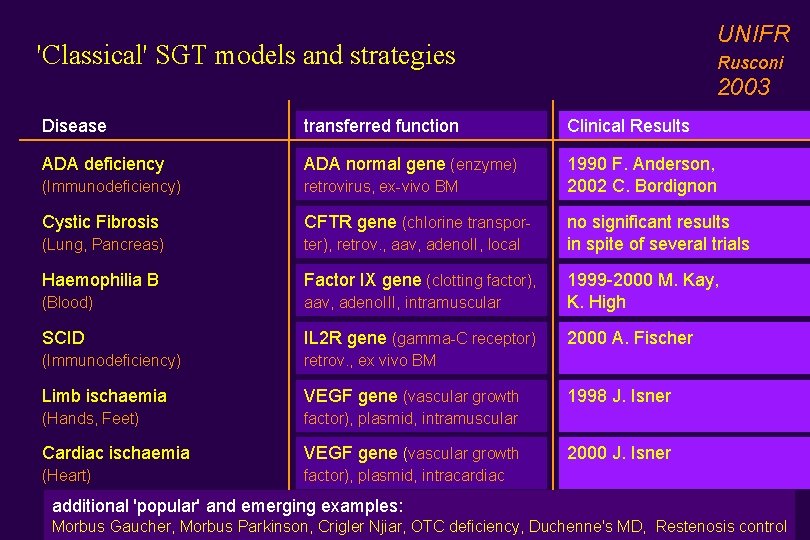
UNIFR 'Classical' SGT models and strategies Rusconi 2003 Disease transferred function Clinical Results ADA deficiency ADA normal gene (enzyme) (Immunodeficiency) retrovirus, ex-vivo BM 1990 F. Anderson, 2002 C. Bordignon Cystic Fibrosis CFTR gene (chlorine transpor- (Lung, Pancreas) ter), retrov. , aav, adeno. II, local Haemophilia B Factor IX gene (clotting factor), (Blood) aav, adeno. III, intramuscular 1999 -2000 M. Kay, K. High SCID IL 2 R gene (gamma-C receptor) 2000 A. Fischer (Immunodeficiency) retrov. , ex vivo BM Limb ischaemia VEGF gene (vascular growth (Hands, Feet) factor), plasmid, intramuscular Cardiac ischaemia VEGF gene (vascular growth (Heart) factor), plasmid, intracardiac no significant results in spite of several trials 1998 J. Isner 2000 J. Isner additional 'popular' and emerging examples: Morbus Gaucher, Morbus Parkinson, Crigler Njiar, OTC deficiency, Duchenne's MD, Restenosis control

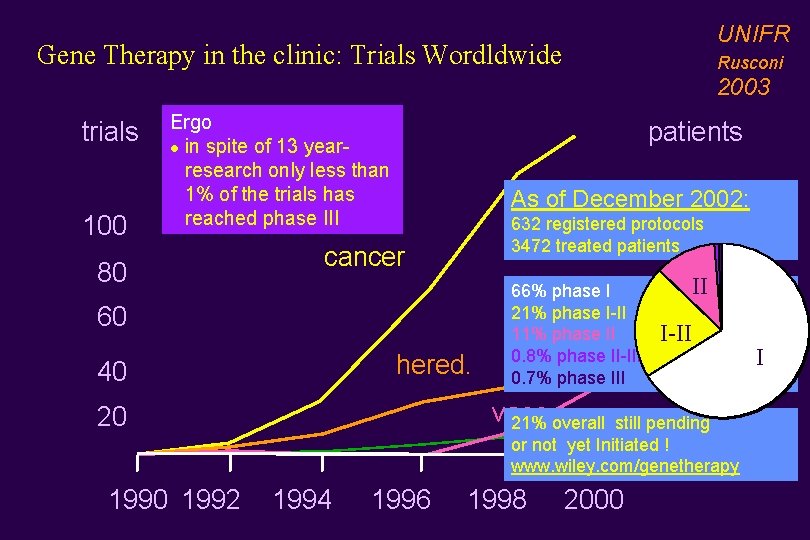
UNIFR Gene Therapy in the clinic: Trials Wordldwide Rusconi 2003 trials 100 Ergo l in spite of 13 year- research only less than 1% of the trials has reached phase III 80 patients As of December 2002: 632 registered protocols 1500 3472 treated patients cancer 60 hered. 40 66% phase I 21% phase I-II 11% phase II 0. 8% phase II-III 0. 7% phase III II 1000 I-II 500 vasc. 21% overall still pending Infect. or not yet Initiated ! 20 www. wiley. com/genetherapy 1990 1992 1994 1996 1998 2000 I

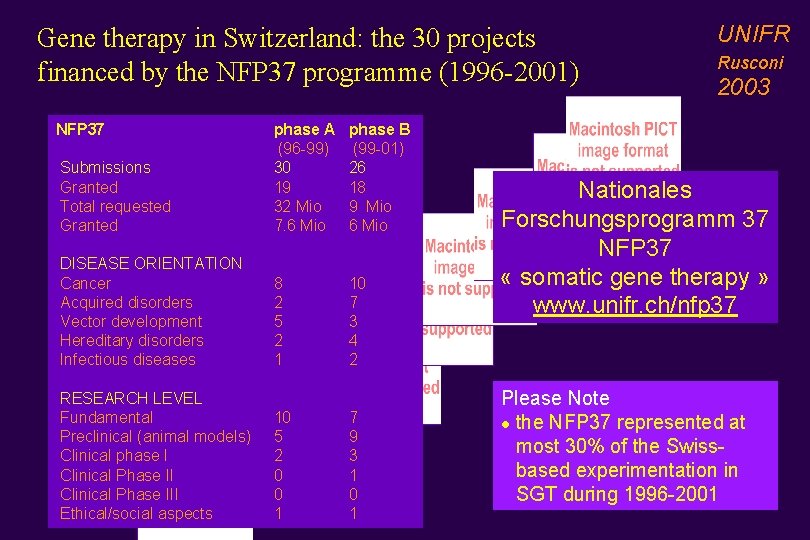
Gene therapy in Switzerland: the 30 projects financed by the NFP 37 programme (1996 -2001) NFP 37 Submissions Granted Total requested Granted DISEASE ORIENTATION Cancer Acquired disorders Vector development Hereditary disorders Infectious diseases RESEARCH LEVEL Fundamental Preclinical (animal models) Clinical phase I Clinical Phase III Ethical/social aspects phase A (96 -99) 30 19 32 Mio 7. 6 Mio phase B (99 -01) 26 18 9 Mio 6 Mio 8 2 5 2 1 10 7 3 4 2 10 5 2 0 0 1 7 9 3 1 0 1 UNIFR Rusconi 2003 Nationales Forschungsprogramm 37 NFP 37 « somatic gene therapy » www. unifr. ch/nfp 37 Please Note l the NFP 37 represented at most 30% of the Swissbased experimentation in SGT during 1996 -2001

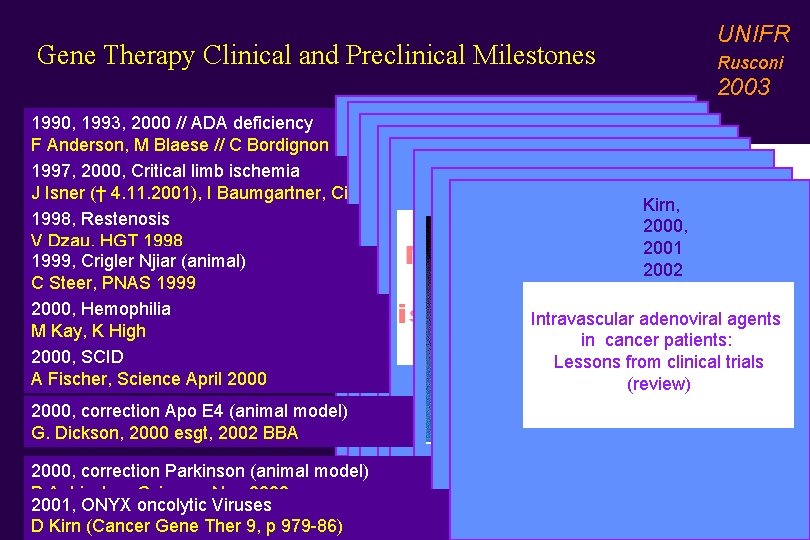
Gene Therapy Clinical and Preclinical Milestones UNIFR Rusconi 2003 Anderson, 1990, 1993, 2000 // ADA deficiency Isner, 1998 Dzau, 1999 F Anderson, M Blaese // C Bordignon Kmiec, 1999 Fischer, 1997, 2000, Critical limb ischemia Dickson, 2000 J Isner († 4. 11. 2001), I Baumgartner, Circulation 1998 Aebischer, 2000 2002 Kirn, 1998, Restenosis 2000, V Dzau, HGT 1998 2001 1999, Crigler Njiar (animal) 2002 C Steer, PNAS 1999 2000, Hemophilia Intravascular adenoviral agents M Kay, K High in cancer patients: 2000, SCID Lessons from clinical trials A Fischer, Science April 2000 (review) Bordignon, 2000 (ESGT, Stockholm) 2000, correction Apo E 4 (animal model) 2002, science 296, 2410 ff) G. Dickson, 2000 esgt, 2002 BBA 2000, correction Parkinson (animal model) P Aebischer, Science, Nov 2000 2001, ONYX oncolytic Viruses D Kirn (Cancer Gene Ther 9, p 979 -86)

Two major SGT frustration cases UNIFR Rusconi 2003 Muscular dystrophy (incidence 1: 3000 newborn males) l l requires persistence of expression extremely large gene (14 kb transcript, 2 mega. BP gene unclear whether regulation necessary unclear at which point disease is irreversible Cystic fibrosis (incidence 1: 2500 newborns) l l luminal attempts failed because of anatomical / biochemical barrier: no receptors, mucus layer large gene that requires probably regulation requires long term regulation unclear at which point disease becomes irreversible Although genes discovered in the 90 ties: l no suitable vector l no satisfactory delivery method

The most feared potential side-effects of gene transfer UNIFR Rusconi 2003 l Immune response to vector l immune response to new or foreign gene product l General toxicity of viral vectors l Adventitious contaminants in recombinant viruses l l Random integration in genome -> insertional mutagenesis (-> cancer risk) Contamination of germ line cells Ergo l Most side effects are still related to the rather primitive state of the vectorology/delivery

Three (four) bitter lessons, but only one treatment-related death so far NY May 5, 1995, R. Crystal: in a trial with adenovirus mediated gene transfer to treat cystic fibrosis (lung) one patient developed a mild pneumonia-like condition and recovered in two weeks. The trial interrupted and many others on hold. UPenn, Sept. 19, 1999, J. Wilson: in a trial with adenovirus mediated gene transfer to treat OTC deficiency (liver) one patient (Jesse Gelsinger) died of a severe septic shock. Many trials were put on hold for several months (years). Paris, Oct 2, 2002, A Fischer: in a trial with retrovirus mediated gene transfer to treat SCID (bone marrow) one patient developed a leukemia-like condition. The trial has been suspended to clarify the issue of insertional mutagenesis, and some trials in US and Germany have been put on hold. Paris, Jan 14, 2003, A Fischer: a second patient of the cohort of 9 comes up with a similar disease than the one reported in october 2002. 30 trials in USA are temporarily suspended UNIFR Rusconi 2003

Public perception problems UNIFR Rusconi 2003 Negative perception of manipulative genetics l l general aversion of genetic manipulation fear of catastrophic scenarios Confusion with other gene-based and non-gene-based technologies l l l stem cell technology human cloning procedures genetically modified food Deception after excessive promises l l hopes reinforced by media spectacularisation and over-simplification deception after non-complied deadline

Other factors that have negatively influenced the public perception and progress of gene therapy l l l UNIFR Rusconi 2003 Naive statements by some good-willing scientists in the early 90 ties Not-so-naive statements by not-so-naive scientists in search of fame Huge amount of money that flowed into the research and development that attracted many incompetent researchers. Concomitance with stock-market euphoria (little attention to realism) Reckless statements or misreporting by greedy scientists or company managers to increase the value of their stock options (memorandum by the ASGT on conflict of interest 2000, www. asgt. . org) Tendency by the media to spectacularise good news and/or bad news Ergo l An explosive cocktail, just like for sports or arts, . . . l the field tends to degenerate as soon as huge amounts of money are involved and when the mass media become interested in it.

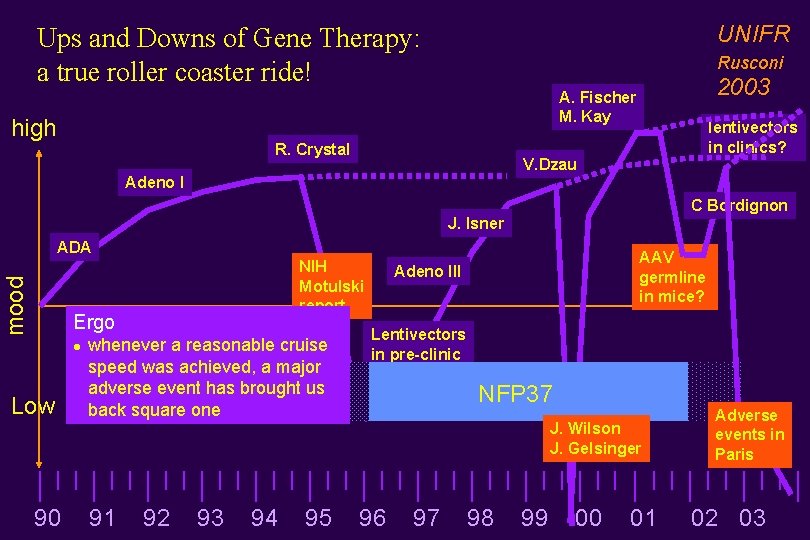
UNIFR Ups and Downs of Gene Therapy: a true roller coaster ride! Rusconi 2003 A. Fischer M. Kay high R. Crystal lentivectors in clinics? V. Dzau Adeno I C Bordignon J. Isner mood ADA NIH Motulski report Ergo l Low whenever a reasonable cruise speed was achieved, a major adverse event has brought us back square one AAV germline in mice? Adeno III Lentivectors in pre-clinic NFP 37 J. Wilson J. Gelsinger 90 91 92 93 94 95 96 97 98 99 00 01 Adverse events in Paris 02 03

Genes, cells, tissue transplants. . . some people fear possible negative developments UNIFR Rusconi 2003 Amelioration instead of therapy? Too High-tech too expensive Bioweapons?

Somatic Gene Therapy is facing fierce competition UNIFR Rusconi 2003 1. Cell Therapy (Stem cells (SC)) l l identified in many tissues cell transfer could be combined with gene transfer there would be no anatomical barriers for gene transfer Selection /amplification of desired transformants Current limitations of SC l l Lack of control on differentiation and trans-determination Difficulties in complex organ-reconstruction 2. Breakthroughs from the small/medium molecules l STI 571 (Glivec) l anti HER 2 (Herceptin) l Si RNA? l. . . Future of SC: l l l Increasing number of SC types will be characterised culturing conditions will be perfectioned May replace in vivo gene transfer for treatment of chronic conditions? V 3. Challengers from the biomechanics world l bone reconstruction l intelligent protheses (stents) l micropumps l artificial organs

UNIFR Conclusions Rusconi 2003 Fundamentally l l l a gene encodes usually more than one function The therapeutic gene transfer in somatic cells must cope with: efficiency, specificity, persistence and toxicity many genes with potential therapeutic value have been identified, and essentially all types of diseases can be treated by gene transfer Vectors and models l l l There is the choice of a certain number of viral and non viral vectors, none of them being generally applicable viral vectors have the advantage of efficiency and nonviral vector the advantage of lower toxicity/danger. viral vectors have the disadvantage of limited packaging and some toxicity, while nonviral vector have the major disadvantage of low efficiency of transfer Clinically l l l over 600 trials and 3500 patients in 12 years only a handful of trials is now reaching phase III Progress further slowed down by periodical pitfalls

UNIFR Perspectives Rusconi 2003 Fundamental level & vectorology l l l the better understanding of gene interactions and networking (functional genomics) could improve the utilisation of gene-based or gene targeted strategies novel paradigms can become available (Si RNA, PNA triplex etc. . . ) specifically integrating gene constructs or artificial chromosomes becoime more realistic Preclinically l l scaling up to larger animal models (dog and monkey) permits better appreciation of dosage requirements new transgenic models may give improved similarities to human diseases Clinically l l Use of recombinant lentiviruses may be imminent Increase of Phase III procedures over the next 5 years First therapeutical applications may be registered within 3 -5 years challenge by other emerging therapies

UNIFR . . . Thanks ! Rusconi 2003 ECPM My collaborators at UNIFR Swiss National Research Foundation Thank you all for the attention, and. . . if you are too shy to ask send an e-mail to: sandro. rusconi@unifr. ch or visit: www. unifr. ch/nfp 37
 2003-1972
2003-1972 1967 1968 1969 1970 1971 1972 1973 1974 1975 1976
1967 1968 1969 1970 1971 1972 1973 1974 1975 1976 1969 1970 1971 1972 1973 1974 1975 1976 1977 1978
1969 1970 1971 1972 1973 1974 1975 1976 1977 1978 Unifr psychologie prüfungen
Unifr psychologie prüfungen Conseil psychologique unifr
Conseil psychologique unifr Sandro pistori
Sandro pistori Sandro botticelli cestello annunciation
Sandro botticelli cestello annunciation Mag oec
Mag oec Sandro sorbi
Sandro sorbi Sandro de cecco
Sandro de cecco Sandro fuzzi
Sandro fuzzi Sandro andreotti
Sandro andreotti Sandro goldoni
Sandro goldoni Schiedsrichter im streit um den zankapfel
Schiedsrichter im streit um den zankapfel Sandro staiano
Sandro staiano Sandro trento
Sandro trento Sandro morasca
Sandro morasca Sandro wenzel
Sandro wenzel Sandro botticelli mariano di vanni dei filipepi
Sandro botticelli mariano di vanni dei filipepi Sandro hawke
Sandro hawke Sandro botticelli achievements
Sandro botticelli achievements Sandro botticelli aportaciones
Sandro botticelli aportaciones Lions
Lions Sandro botticelli alegoria de la primavera
Sandro botticelli alegoria de la primavera Sandro wenzel
Sandro wenzel Banca d'itlia
Banca d'itlia Access matrix
Access matrix Sandro boticelli
Sandro boticelli Sandro salsa
Sandro salsa Since 1975
Since 1975 Cometa west
Cometa west Frank mager
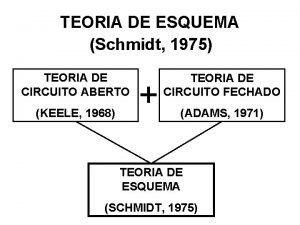
Frank mager Teoria do esquema de schmidt
Teoria do esquema de schmidt Jumlah minimal sampel penelitian eksperimen
Jumlah minimal sampel penelitian eksperimen Gouvernance économique mondiale depuis 1975
Gouvernance économique mondiale depuis 1975 Dan lortie schoolteacher
Dan lortie schoolteacher Modèle de shapero
Modèle de shapero Eesti lauljad
Eesti lauljad 1975-1954
1975-1954 Evaluation of bahrick et al 1975
Evaluation of bahrick et al 1975 Annie leibovitz mick jagger, buffalo, ny (1975)
Annie leibovitz mick jagger, buffalo, ny (1975) Hazardous materials transportation act of 1975
Hazardous materials transportation act of 1975 Loi 75-535 du 30 juin 1975
Loi 75-535 du 30 juin 1975 1962-1975
1962-1975 Gpnm-1975
Gpnm-1975 Tony cragg « stack » 1975
Tony cragg « stack » 1975 1975-1959
1975-1959 1975 personal computer
1975 personal computer Novela de 1939 a 1975
Novela de 1939 a 1975