Unhealthy Alcohol Use in People with HIV Infection



















- Slides: 26

Unhealthy Alcohol Use in People with HIV Infection: The Role of Pharmacotherapy Richard Saitz MD, MPH, FACP, FASAM Professor of Medicine & Epidemiology Boston University Schools of Medicine & Public Health Director, Clinical Addiction, Research and Education (CARE) Unit Boston Medical Center Supported by NIAAA U 24 AA 020801 01, U 01 AA 020802 01, U 01 AA 020793 01

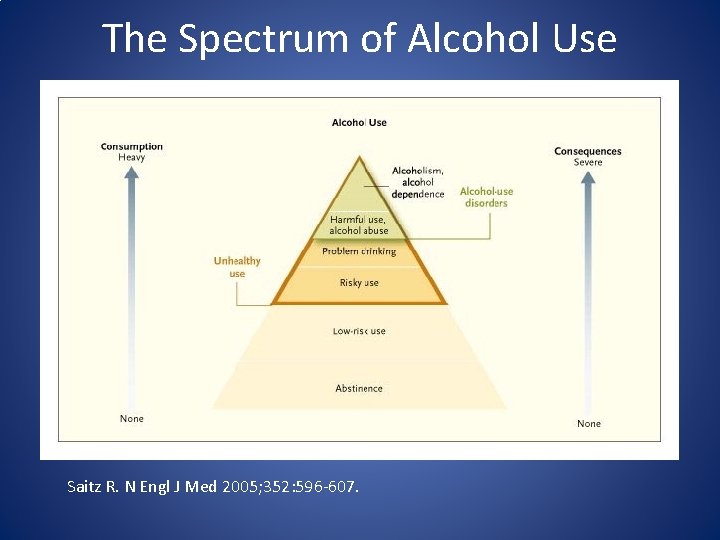
The Spectrum of Alcohol Use Saitz R. N Engl J Med 2005; 352: 596 -607.

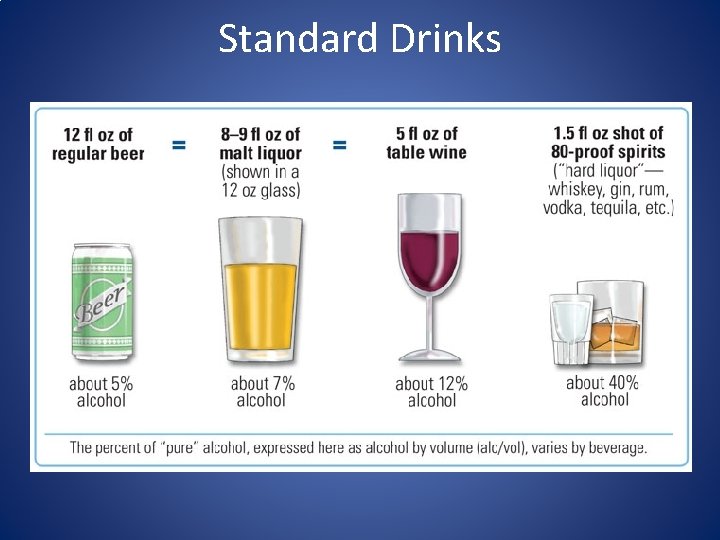
Standard Drinks

Prevalence of Unhealthy Alcohol Use Among People With HIV Cost and Services Utilization Study (n=2864)* 8% HIV Research Network (n=951)† 11% Women’s Interagency HIV Study (n=2770)‡ 14 -24% (11 -year period) Veterans Aging Cohort Study (n=881)** 36% (AUDIT ≥ 8) * Galvan, Bing, Fleishman, et al. J Stud Alcohol. 2002; 63: 179 -186. † Chander, Josephs, Fleishman, et al. HIV Med. 2008; 9: 196 -202. ‡ Cook, Zhu, Belnap, et al. Am J Epidemiol. 2009; 169: 1025 -1032. ** Conigliaro, Gordon, Mc. Ginnis, Rabeneck, Justice. J AIDS. 2003; 33: 521 -525.

Alcohol and ART Non-adherence† • Meta-Analysis of 40 studies and over 25, 000 participants – Risky and dependent drinkers were less likely to be adherent than lower risk drinkers and abstainers (OR 0. 5) Alcohol Use and Risky Sex* • Meta-analysis (n=27 studies) found… – Any alcohol consumption (OR 1. 6) – Problematic drinking (OR 1. 7) – Alcohol use in sexual contexts (OR 2. 0) …significantly associated with unprotected sex among HIVinfected individuals †Hendershot, Stoner, Pantalone, Simoni. J AIDS. 2009. 52: 180 -202. * Shuper, Jonarchi, Irving, Rehm. AIDS Behav. 2009; 13: 1021 -1036.

Alcohol and HIV Disease Progression • Prospective study of 595 HIV+ adults with past or current ‘alcohol problems’ − Among patients not on ART, CD 4 counts averaged 48. 6 cells lower for those with heavy alcohol use v. abstinence (p=0. 03) Samet. JAIDS. 2007; 46: 194 -9.

Screening for Unhealthy Alcohol Use • Single question: How many times in the past year have you had X or more drinks in a day? (where X is 5 for men and 4 for women) A positive test is >1

AUDIT-C (Alcohol Use Disorders Identification Test-Consumption) Question 1: How often do you have a drink containing alcohol? (0) (1) (2) (3) (4) Never Monthly or less 2 to 4 times a month 2 to 3 times a week 4 or more times a week Question 2: How many drinks containing alcohol do you have on a typical day when you are drinking? (0) (1) (2) (3) (4) 1 or 2 3 or 4 5 or 6 7, 8, or 9 10 or more Question 3: How often do you have 4 or more (women) 5 or more (men) drinks on one occasion? (0) (1) (2) (3) (4) Never Less than monthly Monthly Weekly Daily or almost daily >3 in women, >4 in men is positive

Assessing Severity of Unhealthy Use • 10 item AUDIT* – 10 questions, each scored 0 -4 – ≥ 3 (women) ≥ 5 (men) = unhealthy use • NB ≥ 8 is “old” cutoff – ≥ 13 (women) or ≥ 15 (men) consistent with dependence • M. I. N. I. -International Neuropsychiatric Interview (MINI)** – Diagnosis of disorder (abuse/dependence) *http: //whqlibdoc. who. int/hq/2001/who_msd_msb_01. 6 a. pdf and Johnson et al Alcohol Clin Exp Res 2012. Adjust item 3 to 4 drinks for women, 5 for men. Can use 4/6 cutoffs if combined with single-item heavy drinking question [e. g. +score or heavy use]. **https: //medical-outcomes. com/ and Sheehan et al J. Clin Psychiatry, 1998; 59(suppl 20): 22 -33

Addressing Substance Use: Brief Intervention • 10 -15”, empathic • Feedback – Ask permission – Ask what patient thinks of it • Advice (clear) • Goal setting – Negotiate – Menu of options – Support self-efficacy • Follow-up “You are drinking more than is safe for your health. ” “My best medical advice is that you cut down or quit. ” “What do you think? Are you willing to consider making changes? ” Saitz R. N Engl J Med 2005; 352: 596 -607. Samet, Rollnick, Barnes. Arch Intern Med. 1996; 156: 2287 -2293 Boston University Alcohol Clinical Training Program http: //www. bu. edu/act/ and www. mdalcoholtraining. org

Rationale for Pharmacotherapy • Alcohol dependence is a chronic condition • Medications can target neurotransmitters involved in the reinforcing and anxiolytic effects of alcohol use • Beneficial in combination with non-pharmacologic therapy, including counseling and other behavioral therapies • Can reduce relapse and help maintain abstinence

Advantages of HIV care clinic for alcohol pharmacotherapy HIV clinics offer a potential opportunity for the integration of alcohol pharmacotherapy, given: – The long-term care of patients – The integration of a variety of specialty services (e. g. , psychiatric services) – Funding for prescription medications – Intensive case management models that promote outreach to and retention of patients who are often challenging to treat

Patient Selection for Pharmacotherapy • All people with alcohol dependence who are: – currently drinking – experiencing craving or at risk for return to drinking • Considerations – Specific medication contraindications – Alcohol withdrawal– most can safely cut down/quit without medication/inpatient stay – http: //pubs. niaaa. nih. gov/publications/arh 22 -1/05 -12. pdf – Consider risks (age, comorbidity, past seizures or delirium); reassess – Psychosocial support/therapy and follow-up • Primary care medical management* as effective as specialized behavioral therapy** *O’Malley SS et al. Arch Int Med 2003; 163: 1695 -1704. **Anton RF et al. JAMA 2006 May 3; 295: 2003 -17. *Latt NC, et al. Med J Australia 2002; 176: 530 -534.

Current Treatment Options FDA Approved Medications Under Investigation • Naltrexone • Ondansetron – Oral – Injectable • Acamprosate • Disulfiram – Early onset dependence • SSRIs – Late onset dependence – Anxiety/depression • Topiramate – Side effects/tolerability • Baclofen – No effect on liver

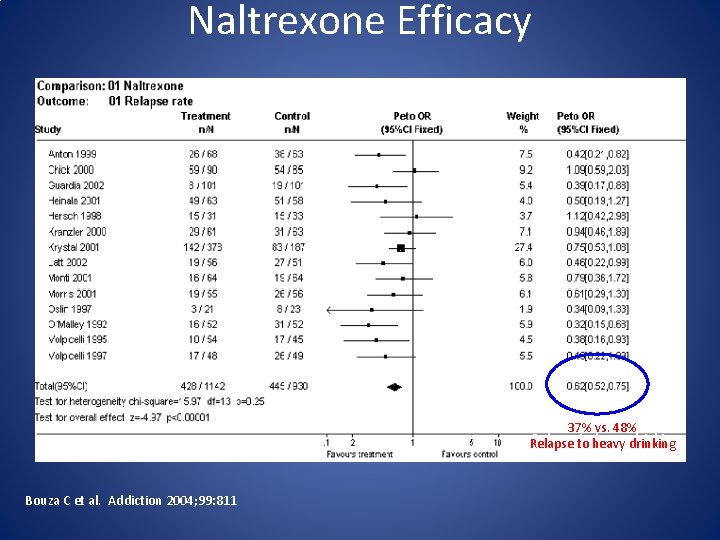
Naltrexone Efficacy 37%vs. 48% Relapse to to heavy drinking Relapse Bouza C et al. Addiction 2004; 99: 811

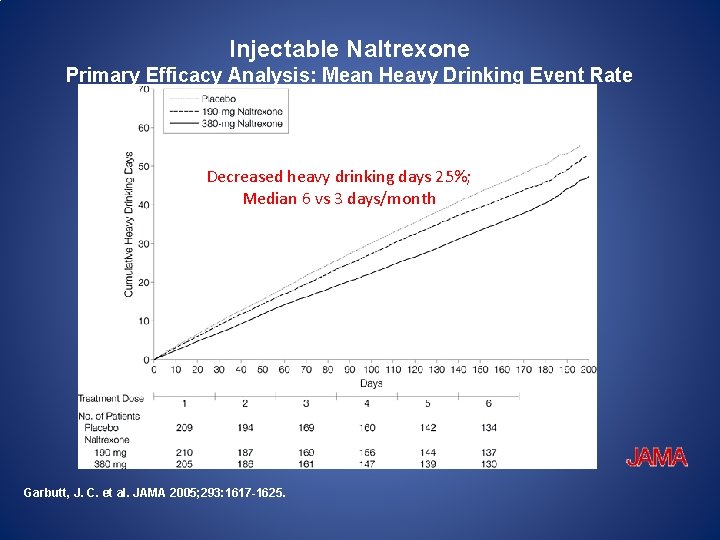
Injectable Naltrexone Primary Efficacy Analysis: Mean Heavy Drinking Event Rate Decreased heavydrinking days 25%; Decreased heavy days 25%; Median 66 vsvs 3 3 days/month Median Garbutt, J. C. et al. JAMA 2005; 293: 1617 -1625.

Prescribing Naltrexone 12. 5 mg/d-->25 mg/d-->50 mg/d (100 mg) or 380 mg IM per month • Main contraindication: opiates • Main side effects: nausea, dizziness • Monitor LFTs post medication initiation • No known drug interactions with antiretroviral therapy

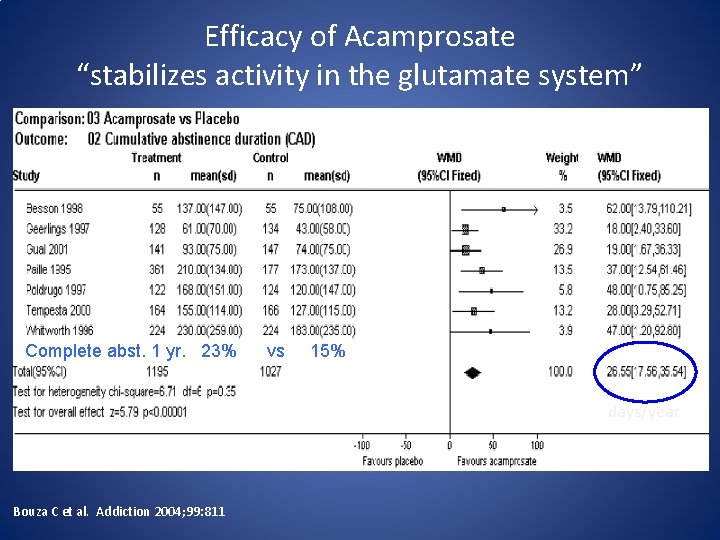
Efficacy of Acamprosate “stabilizes activity in the glutamate system” Complete abst. 1 yr. 23% vs 15% days/year Bouza C et al. Addiction 2004; 99: 811

Prescribing Acamprosate 666 mg tid • Main contraindication: renal insufficiency • Main side effect: diarrhea • No known drug interactions with antiretroviral therapy

Ondansetron • 5 HT 3 antagonist BEFORE AFTER Improvement Johnson BA et al. JAMA 2000; 284: 963 -71 BEFORE AFTER No difference

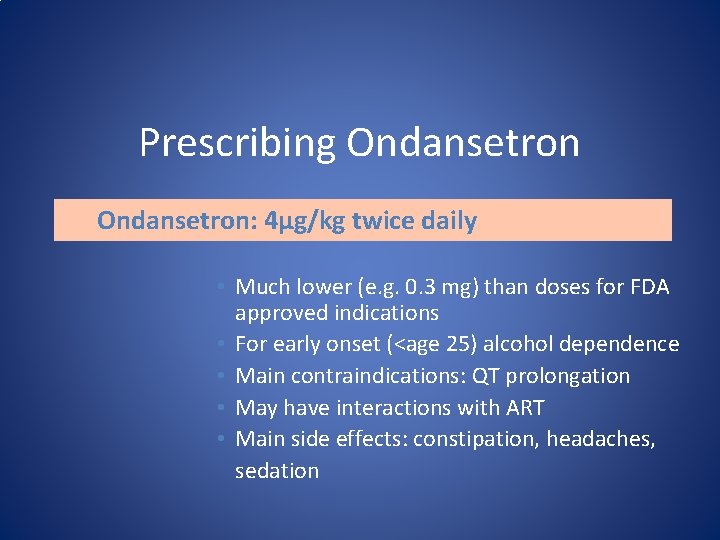
Prescribing Ondansetron: 4µg/kg twice daily • Much lower (e. g. 0. 3 mg) than doses for FDA approved indications • For early onset (<age 25) alcohol dependence • Main contraindications: QT prolongation • May have interactions with ART • Main side effects: constipation, headaches, sedation

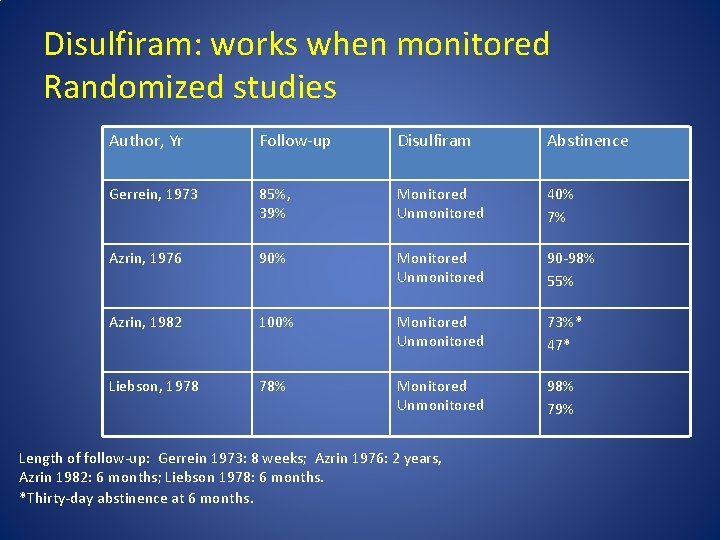
Disulfiram: works when monitored Randomized studies Author, Yr Follow-up Disulfiram Abstinence Gerrein, 1973 85%, 39% Monitored Unmonitored 40% 7% Azrin, 1976 90% Monitored Unmonitored 90 -98% 55% Azrin, 1982 100% Monitored Unmonitored 73%* 47* Liebson, 1978 78% Monitored Unmonitored 98% 79% Length of follow-up: Gerrein 1973: 8 weeks; Azrin 1976: 2 years, Azrin 1982: 6 months; Liebson 1978: 6 months. *Thirty-day abstinence at 6 months.

Prescribing Disulfiram 250 mg/d-->500 mg/d • Main contraindications: recent alcohol use, cognitive impairment, risk of harm from disulfiram--ethanol reaction, drug interactions, rubber, nickel or cobalt allergy • No known drug interactions with antiretroviral therapy – BUT ritonavir liquid and tipranavir caps contain alcohol • Main side effects: hepatitis, neuropathy

Prescribing Tips • Duration of treatment – Most studies 3 -4 months, treatment up to one year, maybe more • Frequency of follow-up – Check LFTs after initiation of naltrexone or disulfiram – Use local clinic resources (nursing) to call patients and discuss adherence to medications and side effects OR have patient return to clinic to discuss • Providing Support – Brief advice can be provided to encourage drinking reduction by physicians, nurses, social workers, counselors – http: //pubs. niaaa. nih. gov/publications/Practitioner/Clinicians. Guide 2005/clinicia ns_guide 19 b_support_p. htm (Google NIAAA Clinicians Guide)

Other Tips • Abstinence desirable but not required for initiation (except disulfiram) • Remind patients that even if they continue to drink alcohol, they should still take their HIV medications and their alcohol medication (unless on disulfiram). • Refer patients to on-line resources (http: //rethinkingdrinking. niaaa. nih. gov/)

Summary • Alcohol use among people with HIV is associated with HIV disease progression, worse medication adherence and increased HIV transmission risk behaviors • Screening and brief intervention for unhealthy alcohol use is a critical component of HIV primary care • Use of pharmacotherapy in persons with alcohol dependence may decrease craving and reduce heavy drinking/promote abstinence
 What is secondary alcohol
What is secondary alcohol Oxidation of a primary alcohol
Oxidation of a primary alcohol Why do the bodys antibodies fail to protect people from hiv
Why do the bodys antibodies fail to protect people from hiv Healthy relationships lesson plan
Healthy relationships lesson plan Healthy and unhealthy relationship scenarios
Healthy and unhealthy relationship scenarios Healthy relationship facts
Healthy relationship facts Define the relationship chapter 14
Define the relationship chapter 14 Unhealthy boundaries definition
Unhealthy boundaries definition Teenager unhealthy lifestyle essay
Teenager unhealthy lifestyle essay The problem of emotionally unhealthy spirituality
The problem of emotionally unhealthy spirituality Healthy vs unhealthy relationships worksheet
Healthy vs unhealthy relationships worksheet Healthy vs unhealthy dating relationships
Healthy vs unhealthy dating relationships Unhealthy symbiotic relationship
Unhealthy symbiotic relationship Military power and control wheel
Military power and control wheel What color are healthy roots
What color are healthy roots Unhealthy relationship scenarios
Unhealthy relationship scenarios Healthy vs unhealthy weight loss
Healthy vs unhealthy weight loss Toxic communication
Toxic communication Quang trung
Quang trung Where did hiv come from
Where did hiv come from Test wiedzy o hiv i aids
Test wiedzy o hiv i aids Hiv index testing steps
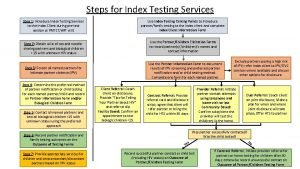
Hiv index testing steps Elemen penilaian prognas snars edisi 1.1
Elemen penilaian prognas snars edisi 1.1 Phdp in hiv
Phdp in hiv Kuchecheudzwa
Kuchecheudzwa Hiv test window period
Hiv test window period Iris syndrome
Iris syndrome