UNEQUAL COUPLING TREE DIAGRAMS SPLITTING DIAGRAMS aka TREE





- Slides: 29

UNEQUAL COUPLING TREE DIAGRAMS SPLITTING DIAGRAMS aka “TREE” DIAGRAMS

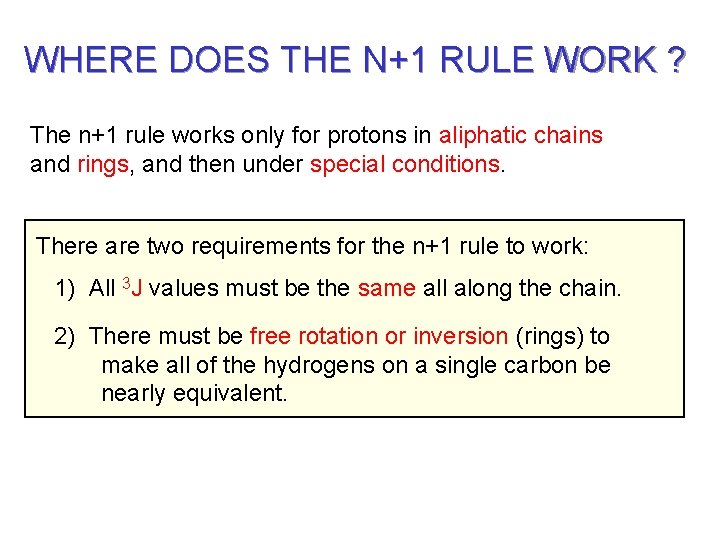
WHERE DOES THE N+1 RULE WORK ? The n+1 rule works only for protons in aliphatic chains and rings, and then under special conditions. There are two requirements for the n+1 rule to work: 1) All 3 J values must be the same all along the chain. 2) There must be free rotation or inversion (rings) to make all of the hydrogens on a single carbon be nearly equivalent.

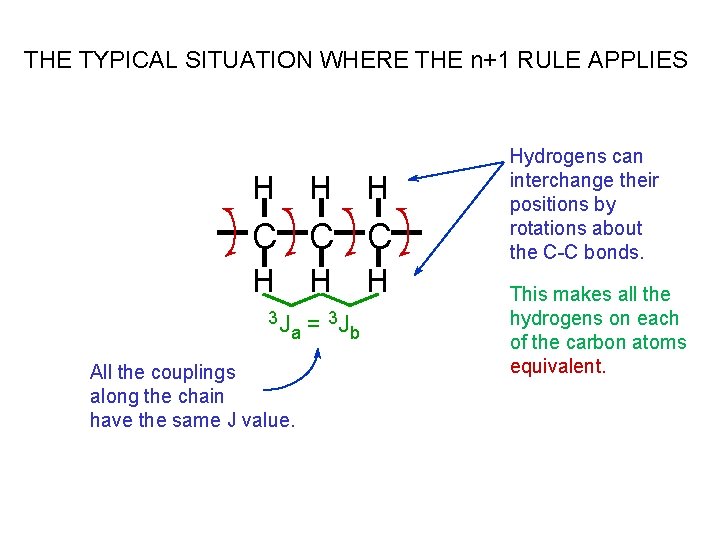
THE TYPICAL SITUATION WHERE THE n+1 RULE APPLIES H H H C C C H H 3 J 3 J = a b All the couplings along the chain have the same J value. H Hydrogens can interchange their positions by rotations about the C-C bonds. This makes all the hydrogens on each of the carbon atoms equivalent.

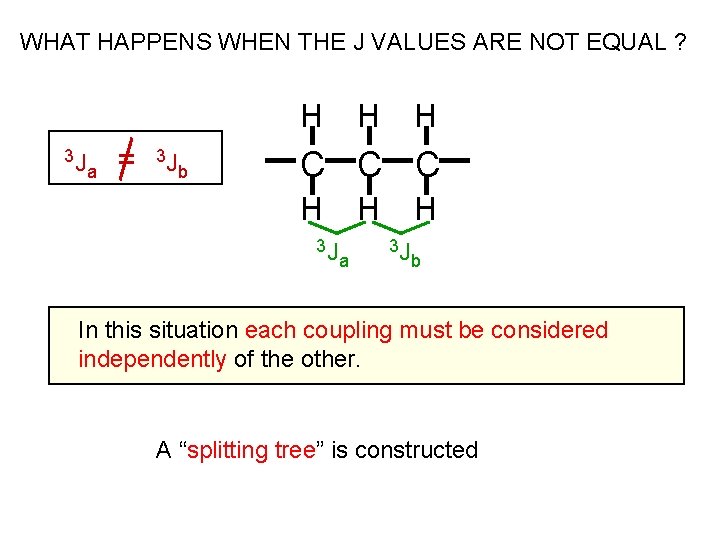
WHAT HAPPENS WHEN THE J VALUES ARE NOT EQUAL ? H 3 J a = 3 J b H H C C C H 3 J H a H 3 J b In this situation each coupling must be considered independently of the other. A “splitting tree” is constructed

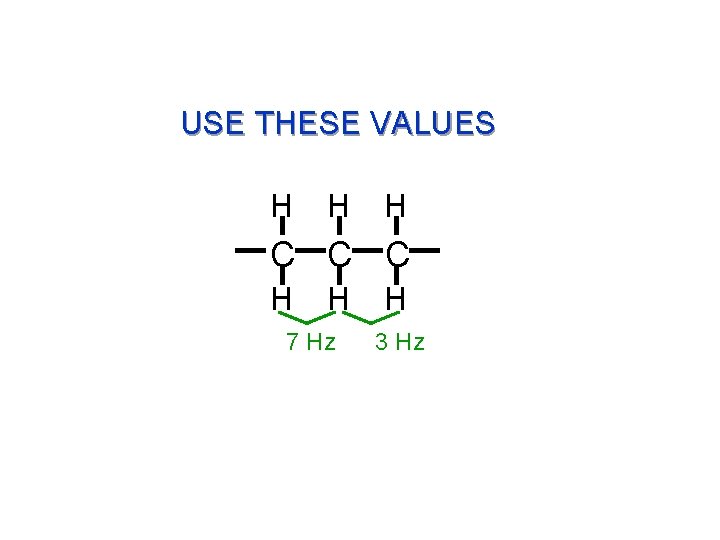
USE THESE VALUES H H H C C C H H 7 Hz H 3 Hz

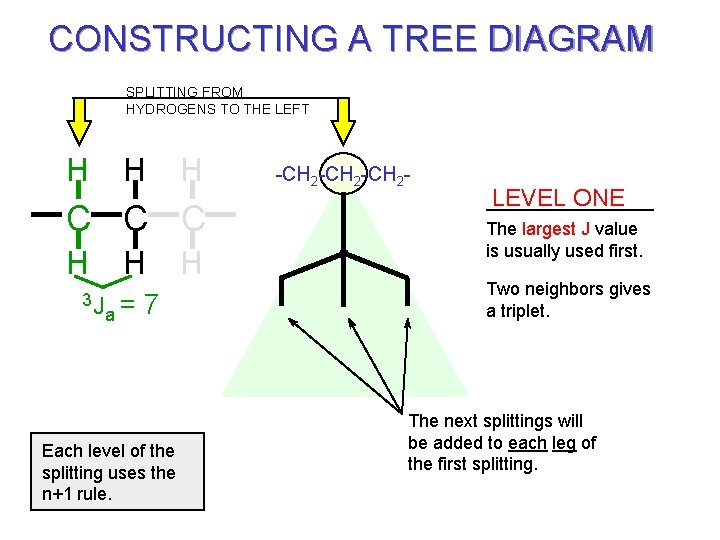
CONSTRUCTING A TREE DIAGRAM SPLITTING FROM HYDROGENS TO THE LEFT H H H C C C H H a= 3 J 7 Each level of the splitting uses the n+1 rule. H -CH 2 -CH 2 - LEVEL ONE The largest J value is usually used first. Two neighbors gives a triplet. The next splittings will be added to each leg of the first splitting.

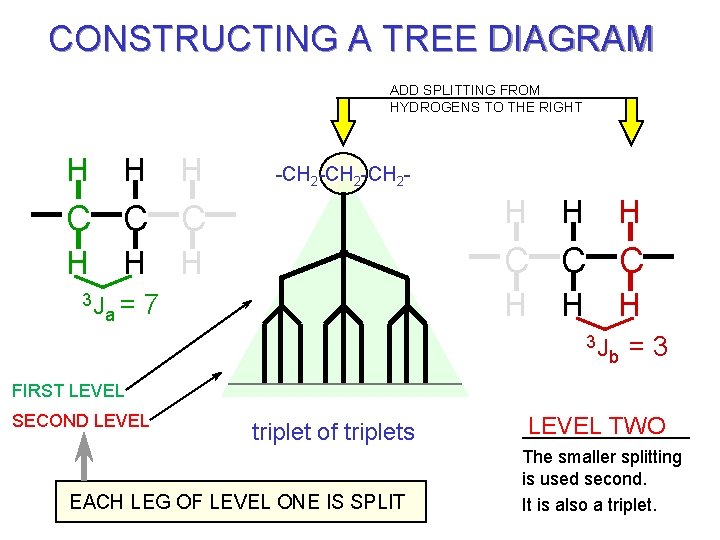
CONSTRUCTING A TREE DIAGRAM ADD SPLITTING FROM HYDROGENS TO THE RIGHT H H H -CH 2 -CH 2 - C C C H H C C C H a= 3 J H H 7 H H 3 J b =3 FIRST LEVEL SECOND LEVEL triplet of triplets EACH LEG OF LEVEL ONE IS SPLIT LEVEL TWO The smaller splitting is used second. It is also a triplet.

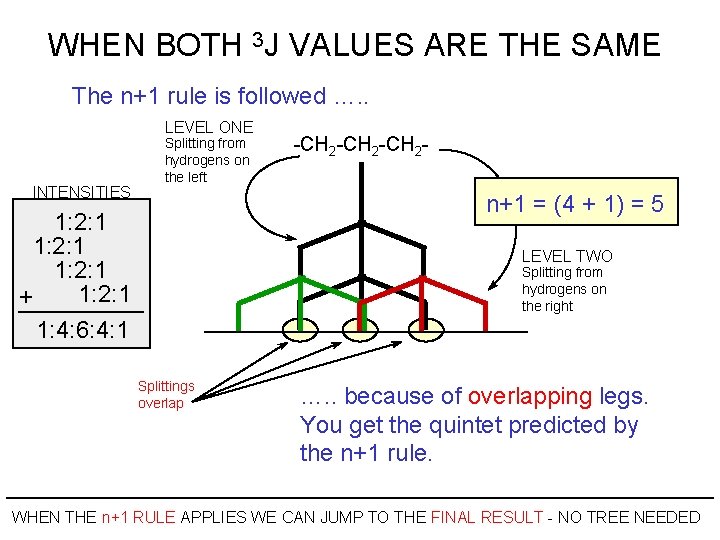
WHEN BOTH 3 J VALUES ARE THE SAME The n+1 rule is followed …. . LEVEL ONE INTENSITIES Splitting from hydrogens on the left -CH 2 -CH 2 - n+1 = (4 + 1) = 5 1: 2: 1 + 1: 4: 6: 4: 1 LEVEL TWO Splitting from hydrogens on the right Splittings overlap …. . because of overlapping legs. You get the quintet predicted by the n+1 rule. WHEN THE n+1 RULE APPLIES WE CAN JUMP TO THE FINAL RESULT - NO TREE NEEDED

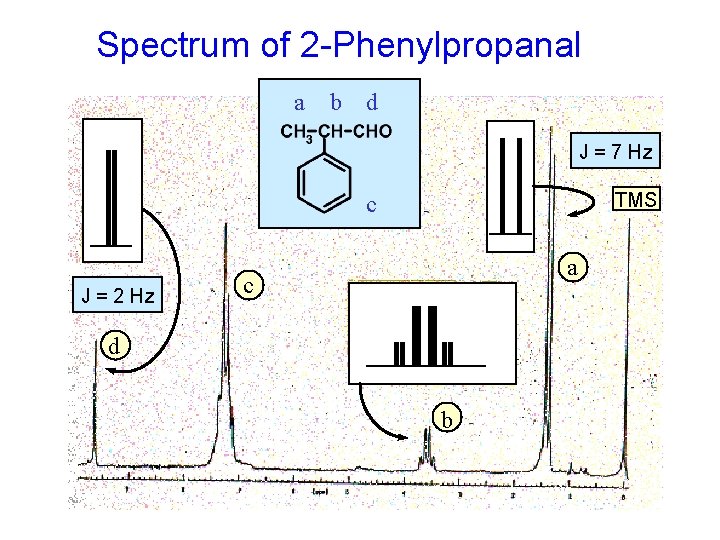
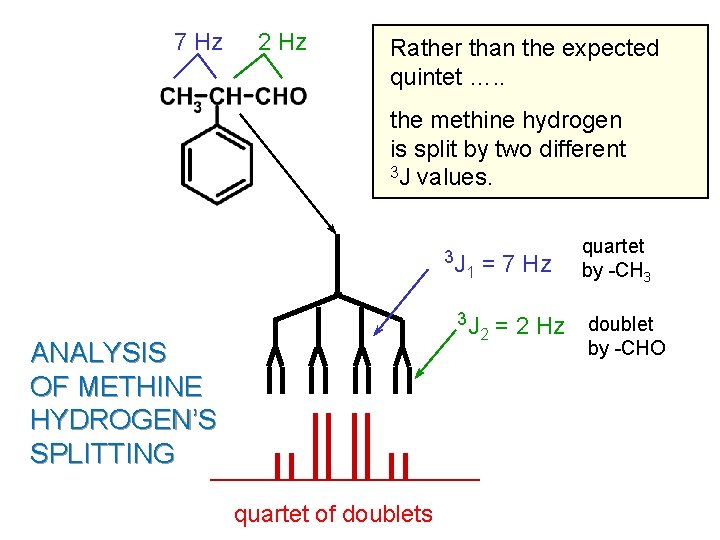
2 -PHENYLPROPANAL A case where there are unequal J values.

Spectrum of 2 -Phenylpropanal a b d J = 7 Hz TMS c J = 2 Hz a c d b

7 Hz 2 Hz Rather than the expected quintet …. . the methine hydrogen is split by two different 3 J values. 3 J = 7 Hz 1 3 J 2 ANALYSIS OF METHINE HYDROGEN’S SPLITTING quartet of doublets quartet by -CH 3 = 2 Hz doublet by -CHO

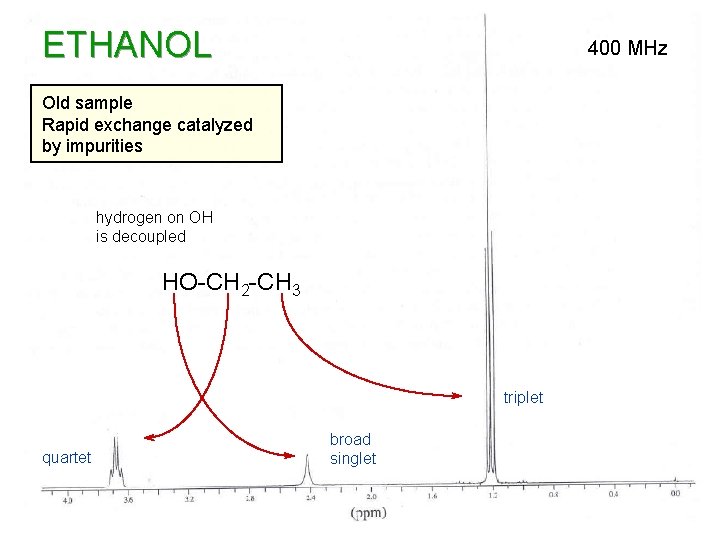
PURE ETHANOL

ETHANOL 400 MHz Old sample Rapid exchange catalyzed by impurities hydrogen on OH is decoupled HO-CH 2 -CH 3 triplet quartet broad singlet

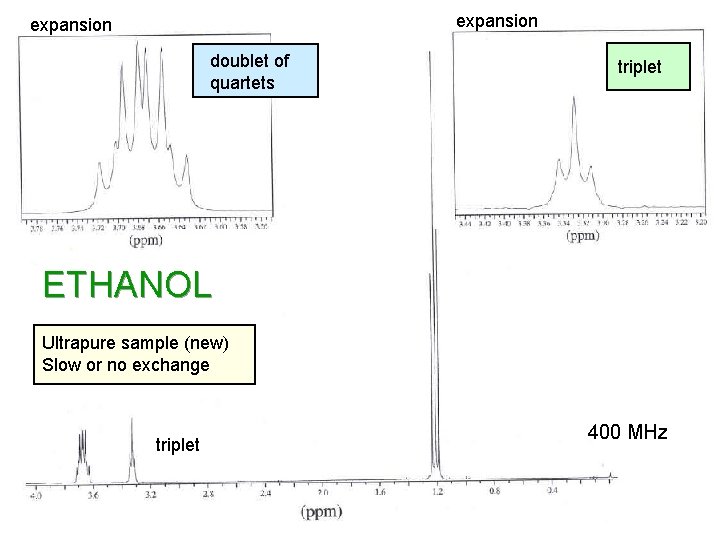
expansion doublet of quartets triplet ETHANOL Ultrapure sample (new) Slow or no exchange triplet 400 MHz

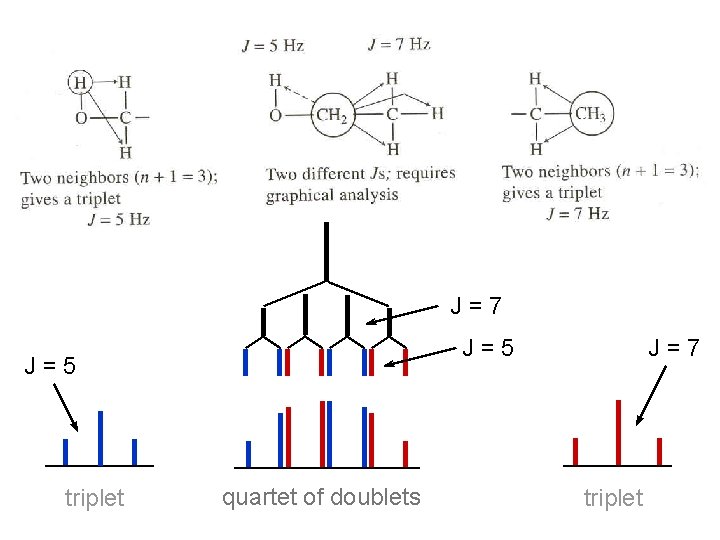
J=7 J=5 triplet quartet of doublets J=7 triplet

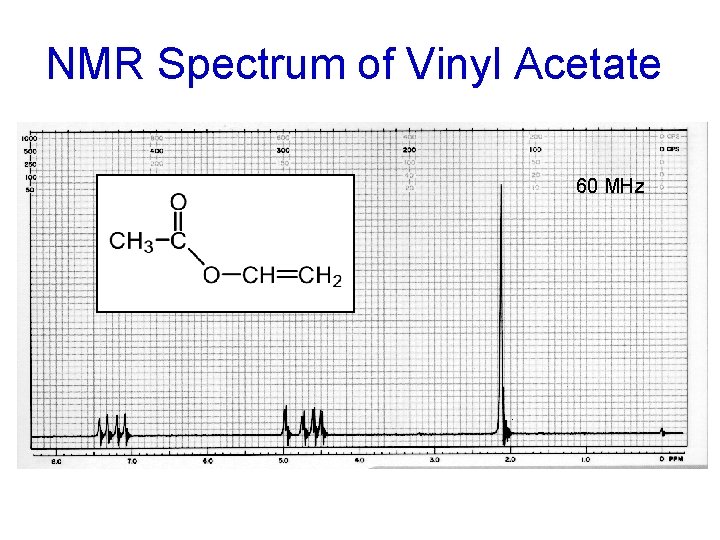
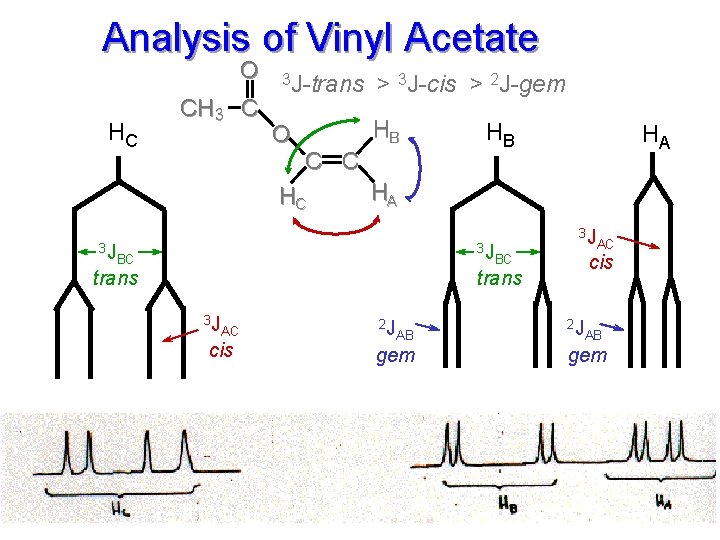
VINYL ACETATE ALKENE HYDROGENS

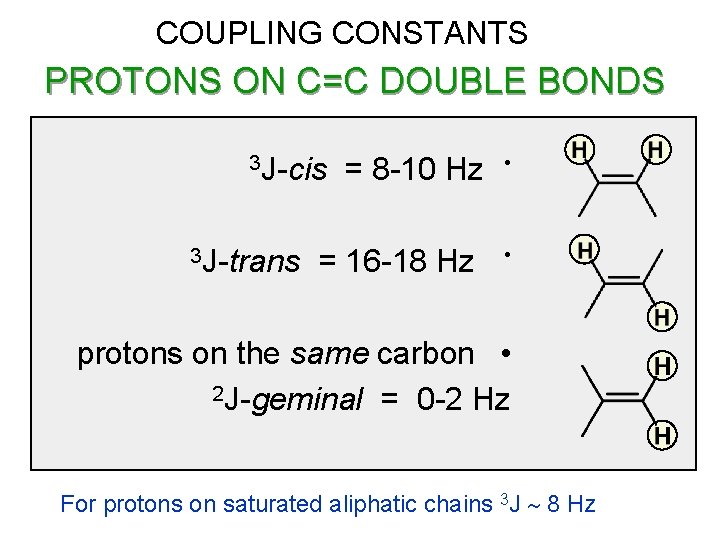
COUPLING CONSTANTS PROTONS ON C=C DOUBLE BONDS 3 J-cis 3 J-trans = 8 -10 Hz = 16 -18 Hz • • protons on the same carbon • 2 J-geminal = 0 -2 Hz For protons on saturated aliphatic chains 3 J ~ 8 Hz

NMR Spectrum of Vinyl Acetate 60 MHz

Analysis of Vinyl Acetate O HC CH 3 C 3 J-trans > 3 J-cis > 2 J-gem HB O C C HC HB HA HA 3 J 3 J AC 3 J BC cis BC trans 3 J AC cis 2 J 2 J gem AB AB

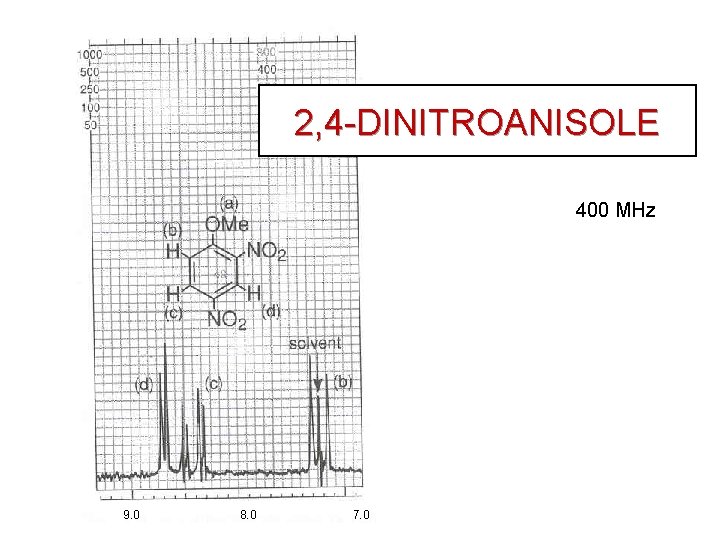
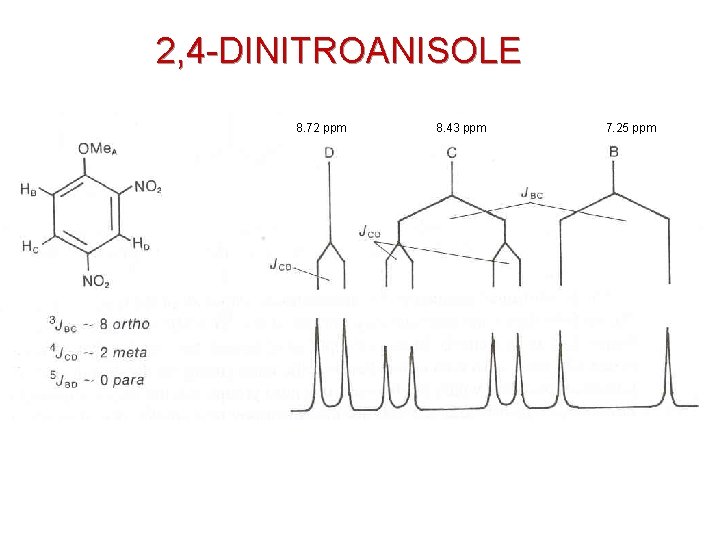
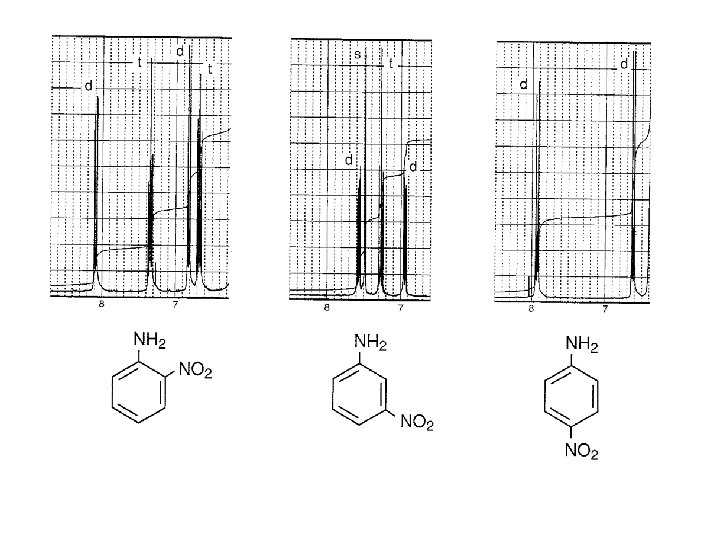
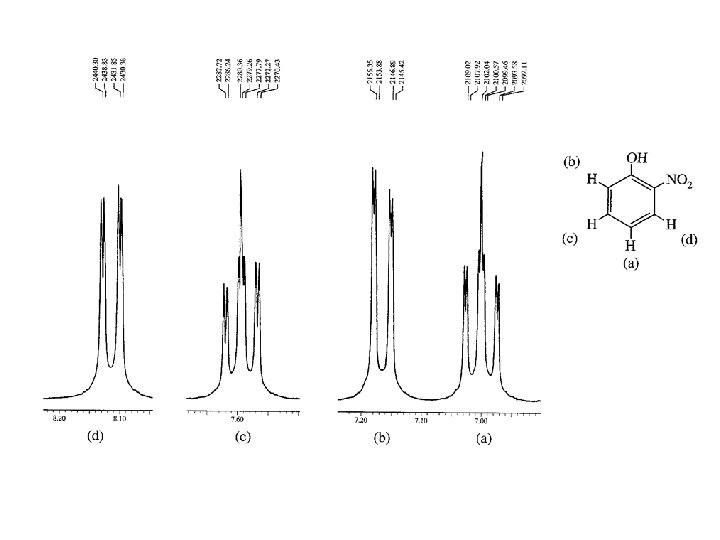
2, 4 -DINITROANISOLE BENZENE HYDROGENS

2, 4 -DINITROANISOLE 400 MHz 9. 0 8. 0 7. 0

2, 4 -DINITROANISOLE 8. 72 ppm 8. 43 ppm 7. 25 ppm







END