UNDERSTANDING THE STRUCTURAL AND PHYSICAL BASIS OF SELENIUM

UNDERSTANDING THE STRUCTURAL AND PHYSICAL BASIS OF SELENIUM BASED SEMICONDUCTOR Professor Zainal Abidin Talib Dr. Josephine Liew Ying Chyi Professor W. Mahmood Mat Yunus
INTRODUCTION
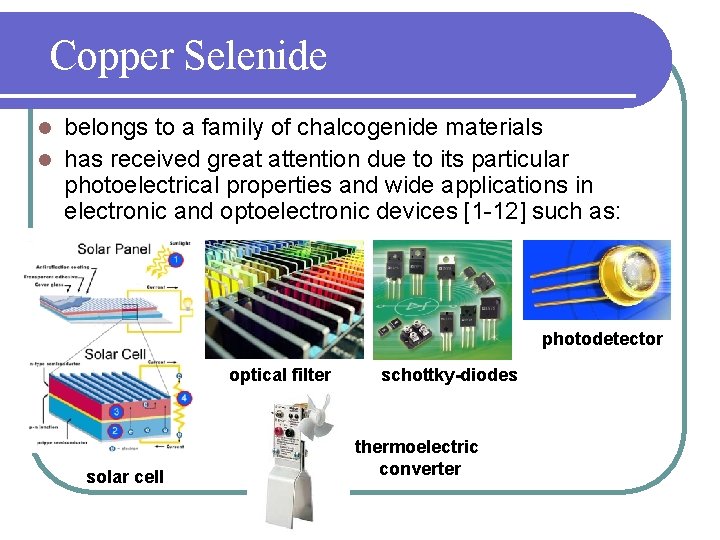
Copper Selenide belongs to a family of chalcogenide materials l has received great attention due to its particular photoelectrical properties and wide applications in electronic and optoelectronic devices [1 -12] such as: l photodetector optical filter solar cell schottky-diodes thermoelectric converter
l The attraction of this binary material also depends on its feasibility to use as a precursor material to incorporate indium or other elements made available and lead to formation of ternary compound such as copper indium diselenide (Cu. In. Se 2) or other multinary material for thin film solar cell application [13 -17]. l It has a wide range of stoichiometric compositions (Cu. Se, Cu 2 Se, Cu 3 Se 2, Cu 7 Se 4, Cu 5 Se 4, Cu. Se 2) and non-stoichiometric composition (Cu 2 -x. Se) [8, 18] l Copper selenide can be constructed into several well documented crystallographic (phases and structural) forms such as orthorhombic [17, 19 -21], monoclinic [22], cubic [21 -24], tetragonal [17, 21], hexagonal [24 -26] etc depending on their compositions form by various preparation technique [22, 27].
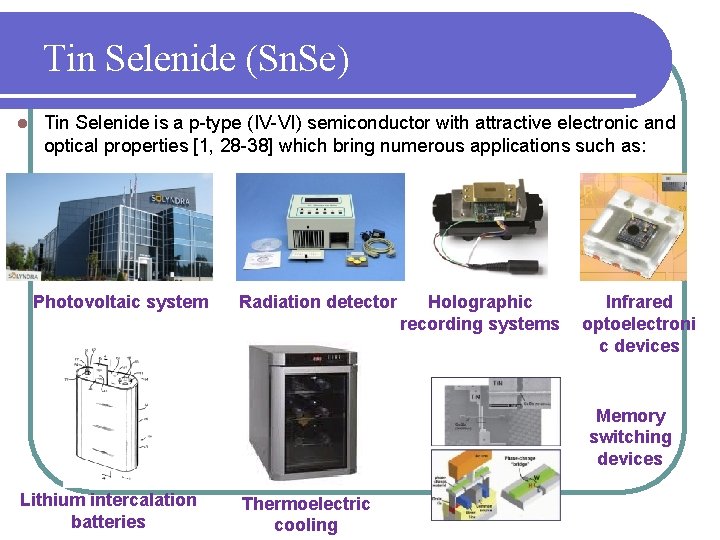
Tin Selenide (Sn. Se) l Tin Selenide is a p-type (IV-VI) semiconductor with attractive electronic and optical properties [1, 28 -38] which bring numerous applications such as: Photovoltaic system Radiation detector Holographic recording systems Infrared optoelectroni c devices Memory switching devices Lithium intercalation batteries Thermoelectric cooling

l l l Tin Selenide are classified as narrow-gap semiconductors (bandgap 1 – 2 e. V) and are capable of absorbing major part of solar energy for photovoltaic applications[1, 28, 39, 40]. Tin monoselenide is a p-type semiconductor with an orthorhombic structure. The tin(II) selenide crystals are construct by tightly bound layers which formed by double planes with zigzag chains of tin and selenium atoms[41]. The highly layered structure, typical of all orthorhombic chlacogenide crystals, causes a strong anisotropy of physical properties of tin(II) selenide. Because of their anisotropic character, the tin (II) selenide chalcogenides becomes an attractive layered compounds, and can be used as cathode materials in lithium intercalation batteries[42].
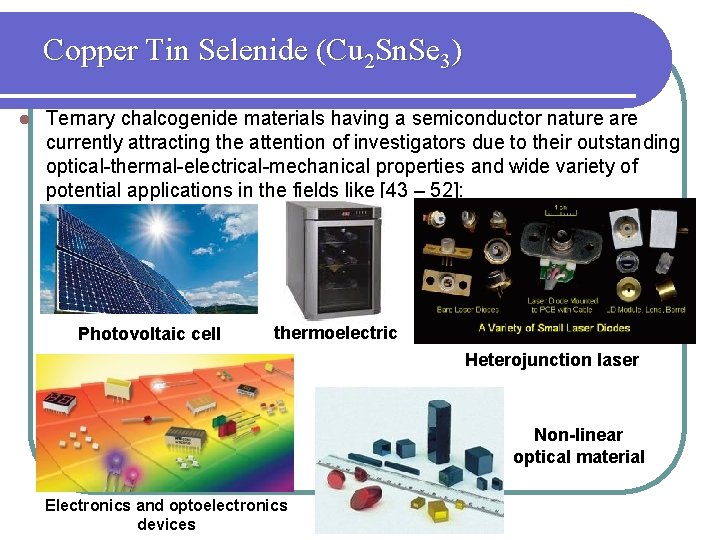
Copper Tin Selenide (Cu 2 Sn. Se 3) l Ternary chalcogenide materials having a semiconductor nature are currently attracting the attention of investigators due to their outstanding optical-thermal-electrical-mechanical properties and wide variety of potential applications in the fields like [43 – 52]: Photovoltaic cell thermoelectric Heterojunction laser Non-linear optical material Electronics and optoelectronics devices
l The study of these materials is important since their band gap and lattice parameters can be varied by changing the cation composition, low melting temperature at around 690 o. C, high mean atomic weight and high refractive indices [51, 53 – 55]. l Copper indium diselenide (Cu. In. Se 2) currently is one of the main compound used in photovoltaic application. l However, indium are not cheap, therefore replacing indium with tin will potentially be cost-competitive as tin supply are more abundant and cheaper. l Preparation of copper tin selenide system will lead to lower production cost and making supply situation more stable.
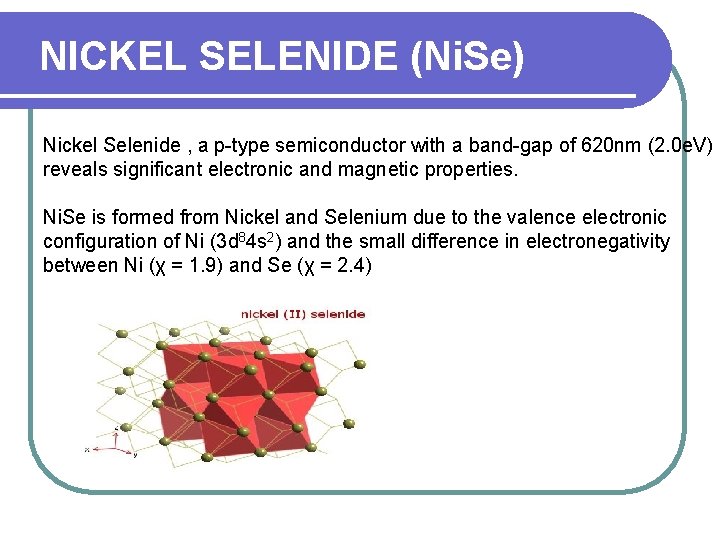
NICKEL SELENIDE (Ni. Se) Nickel Selenide , a p-type semiconductor with a band-gap of 620 nm (2. 0 e. V) reveals significant electronic and magnetic properties. Ni. Se is formed from Nickel and Selenium due to the valence electronic configuration of Ni (3 d 84 s 2) and the small difference in electronegativity between Ni (χ = 1. 9) and Se (χ = 2. 4)
IRON SELENIDE (Fe. Se 2) • Semiconductors • Potential material for future applications in magnetoelectronics • Potential material for future applications in optoelectronic devices
ZINC SELENIDE (Zn. Se) • Zn. Se is good candidates for applications in various optoelectronic devices such as light emitting diodes (LED), semiconductor laser and photodetector. • This is because of the nanometer size structure makes the electronic energy state discrete. • When the diameter of nanocrystals is decreased, the energy separation and quantum effect will be enhanced.
OBJECTIVE l Fabrication of selenium based semiconductor (Cu. Se, Sn. Se, Ni. Se 2, Cu 2 Sn. Se 3) in powder form (compositional analysis) and thin film (deposition condition analysis). l Optical, electrical and thermal properties characterization of the Se based semiconductor. l Evaluate the temperature dependence of the selenium based semiconductor from the observation of structural, electrical, optical and thermal properties changes at various temperature.

IMPORTANT OF STUDIES l It is evident that for the future well-being of nations, a supply of energy based on a renewable source which is economically and environmentally acceptable has to be developed. l Successful production of an efficient metal chalcogenide solar cell and modules requires the coupling of fabrication techniques with a basic understanding of the devices. l There is a need to develop a greater fundamental sciences and engineering basis for the selenium based semiconductor material devices and processing requirement. l In this work, we have fill the information gap on literature about the fundamental study of the structural, electrical, thermal and optical studies in polycrystalline Cu. Se, Sn. Se, Ni. Se 2, Zn. Se, Fe. Se 2 and Cu 2 Sn. Se 3 material. l This fundamental knowledge will guide us to find out the fabrication and design parameters, which are imposed by current technology, material specifications and irradiation conditions to maximize the solar cell efficiency.

Sample Preparation Powder preparation – Chemical Precipitation Technique Pellet preparation – Moulding Thin film preparation – Thermal Evaporation Technique
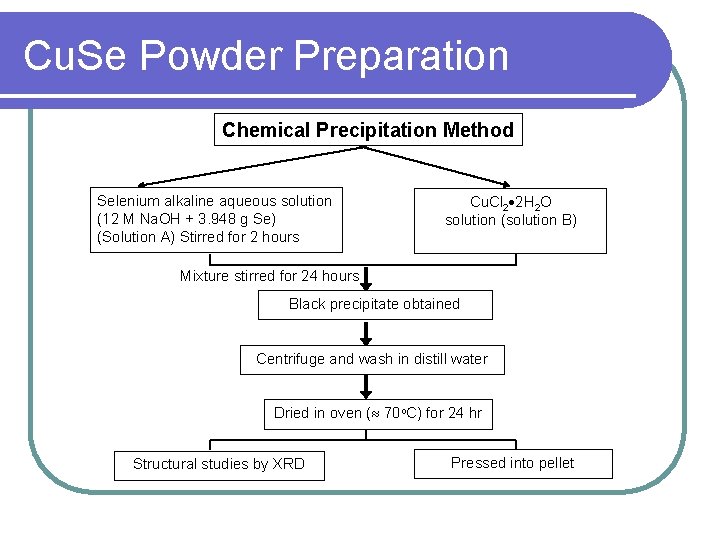
Cu. Se Powder Preparation Chemical Precipitation Method Selenium alkaline aqueous solution (12 M Na. OH + 3. 948 g Se) (Solution A) Stirred for 2 hours Cu. Cl 2 2 H 2 O solution (solution B) Mixture stirred for 24 hours Black precipitate obtained Centrifuge and wash in distill water Dried in oven ( 70 o. C) for 24 hr Structural studies by XRD Pressed into pellet
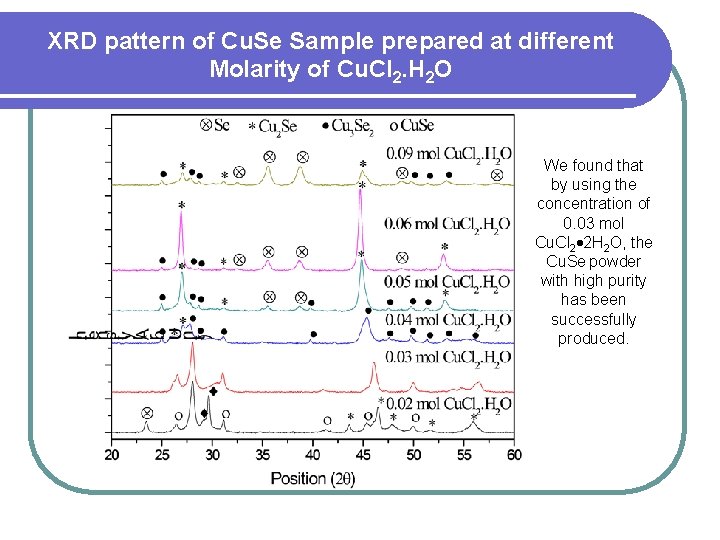
XRD pattern of Cu. Se Sample prepared at different Molarity of Cu. Cl 2. H 2 O We found that by using the concentration of 0. 03 mol Cu. Cl 2 2 H 2 O, the Cu. Se powder with high purity has been successfully produced.
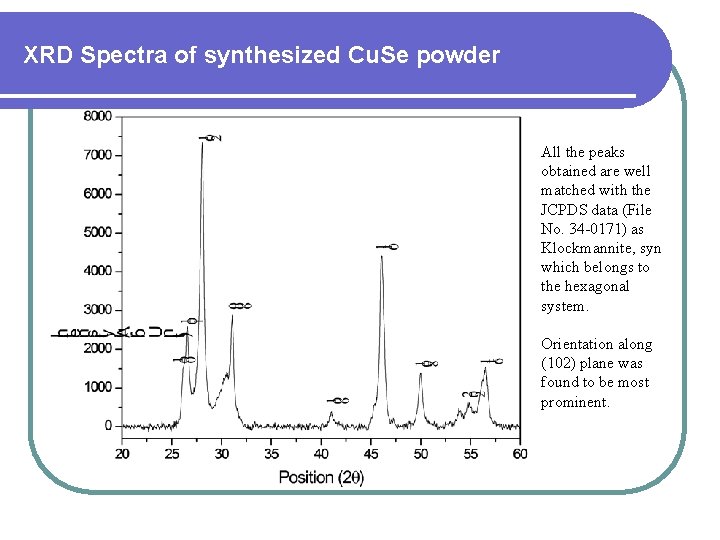
XRD Spectra of synthesized Cu. Se powder All the peaks obtained are well matched with the JCPDS data (File No. 34 -0171) as Klockmannite, syn which belongs to the hexagonal system. Orientation along (102) plane was found to be most prominent.
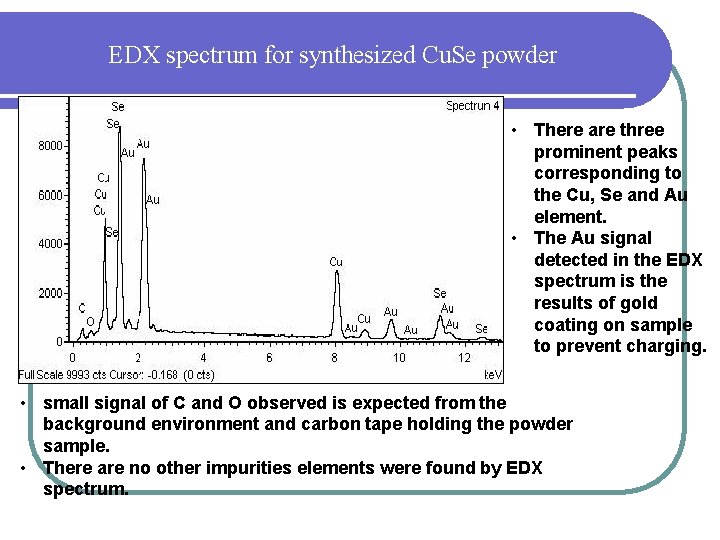
EDX spectrum for synthesized Cu. Se powder • There are three prominent peaks corresponding to the Cu, Se and Au element. • The Au signal detected in the EDX spectrum is the results of gold coating on sample to prevent charging. • small signal of C and O observed is expected from the background environment and carbon tape holding the powder sample. • There are no other impurities elements were found by EDX spectrum.
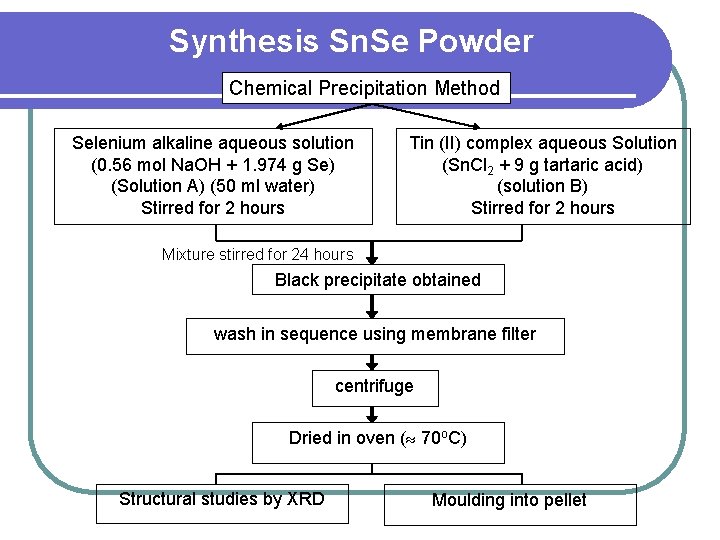
Synthesis Sn. Se Powder Chemical Precipitation Method Selenium alkaline aqueous solution (0. 56 mol Na. OH + 1. 974 g Se) (Solution A) (50 ml water) Stirred for 2 hours Tin (II) complex aqueous Solution (Sn. Cl 2 + 9 g tartaric acid) (solution B) Stirred for 2 hours Mixture stirred for 24 hours Black precipitate obtained wash in sequence using membrane filter centrifuge Dried in oven ( 70 o. C) Structural studies by XRD Moulding into pellet
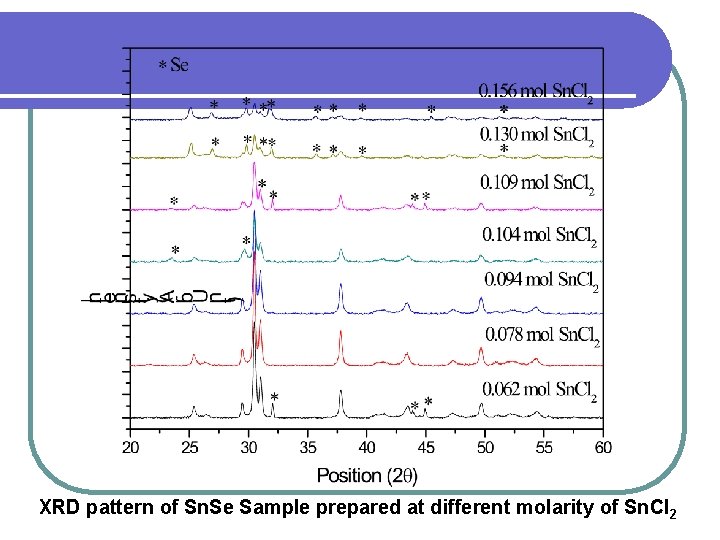
XRD pattern of Sn. Se Sample prepared at different molarity of Sn. Cl 2
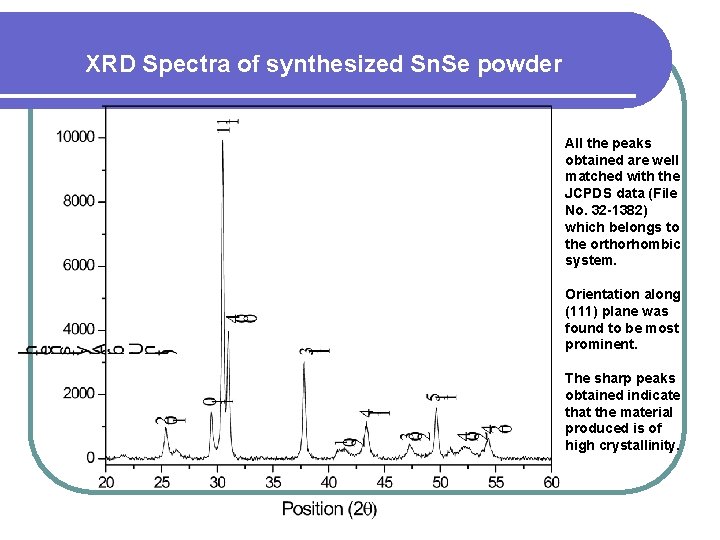
XRD Spectra of synthesized Sn. Se powder All the peaks obtained are well matched with the JCPDS data (File No. 32 -1382) which belongs to the orthorhombic system. Orientation along (111) plane was found to be most prominent. The sharp peaks obtained indicate that the material produced is of high crystallinity.
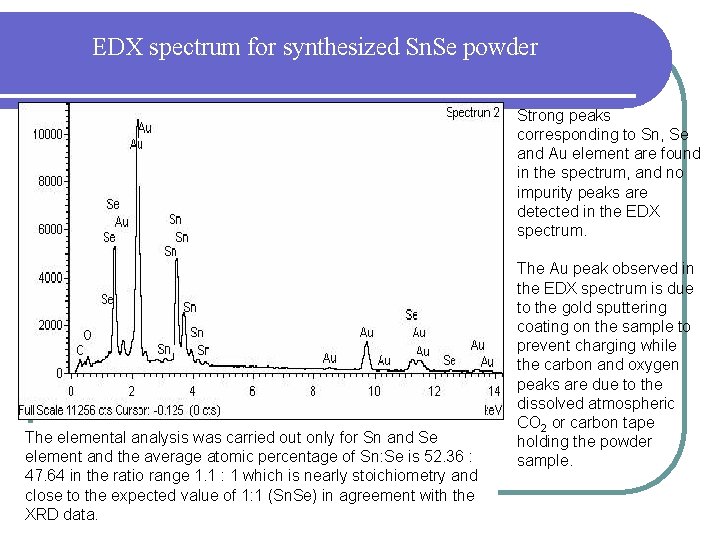
EDX spectrum for synthesized Sn. Se powder Strong peaks corresponding to Sn, Se and Au element are found in the spectrum, and no impurity peaks are detected in the EDX spectrum. The elemental analysis was carried out only for Sn and Se element and the average atomic percentage of Sn: Se is 52. 36 : 47. 64 in the ratio range 1. 1 : 1 which is nearly stoichiometry and close to the expected value of 1: 1 (Sn. Se) in agreement with the XRD data. The Au peak observed in the EDX spectrum is due to the gold sputtering coating on the sample to prevent charging while the carbon and oxygen peaks are due to the dissolved atmospheric CO 2 or carbon tape holding the powder sample.
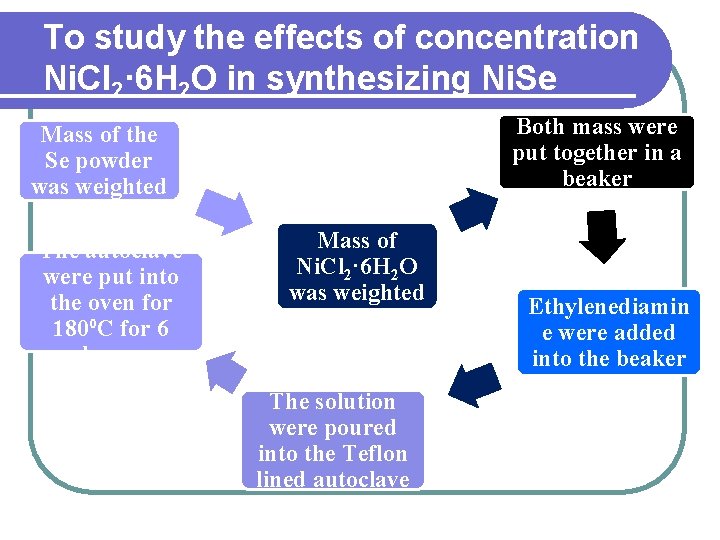
To study the effects of concentration Ni. Cl 2· 6 H 2 O in synthesizing Ni. Se Both mass were put together in a beaker Mass of the Se powder was weighted The autoclave were put into the oven for 180⁰C for 6 hours Mass of Ni. Cl 2· 6 H 2 O was weighted The solution were poured into the Teflon lined autoclave Ethylenediamin e were added into the beaker
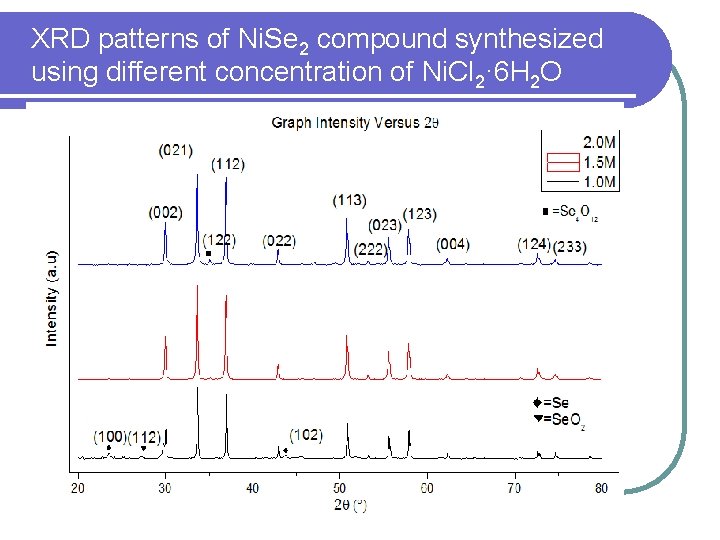
XRD patterns of Ni. Se 2 compound synthesized using different concentration of Ni. Cl 2· 6 H 2 O
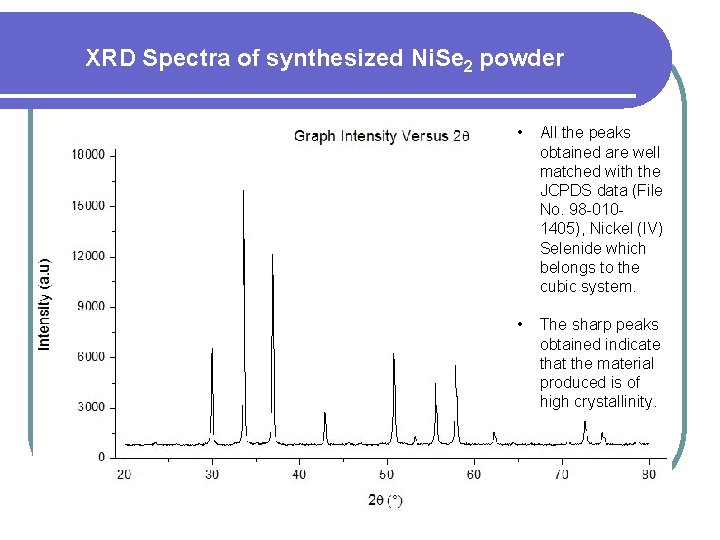
XRD Spectra of synthesized Ni. Se 2 powder • All the peaks obtained are well matched with the JCPDS data (File No. 98 -0101405), Nickel (IV) Selenide which belongs to the cubic system. • The sharp peaks obtained indicate that the material produced is of high crystallinity.
Synthesis of Zn. Se Compound

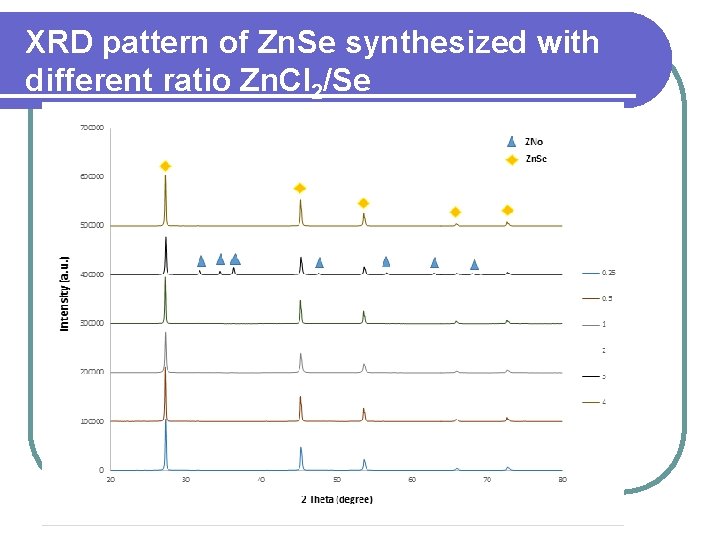
XRD pattern of Zn. Se synthesized with different ratio Zn. Cl 2/Se
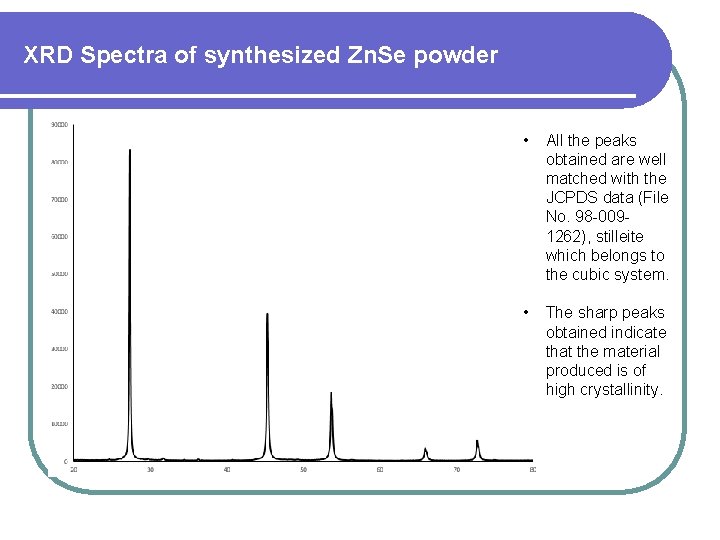
XRD Spectra of synthesized Zn. Se powder • All the peaks obtained are well matched with the JCPDS data (File No. 98 -0091262), stilleite which belongs to the cubic system. • The sharp peaks obtained indicate that the material produced is of high crystallinity.
Synthesis of Fe. Se 2 sample
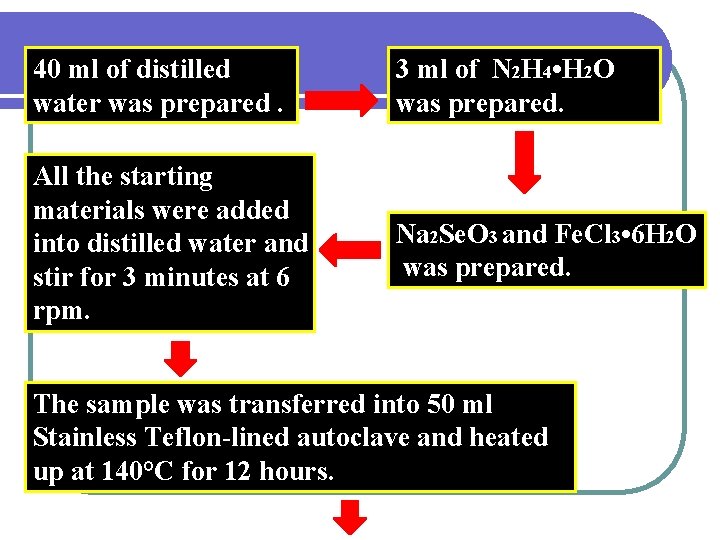
40 ml of distilled water was prepared. 3 ml of N 2 H 4 • H 2 O was prepared. All the starting materials were added into distilled water and stir for 3 minutes at 6 rpm. Na 2 Se. O 3 and Fe. Cl 3 • 6 H 2 O was prepared. The sample was transferred into 50 ml Stainless Teflon-lined autoclave and heated up at 140°C for 12 hours.
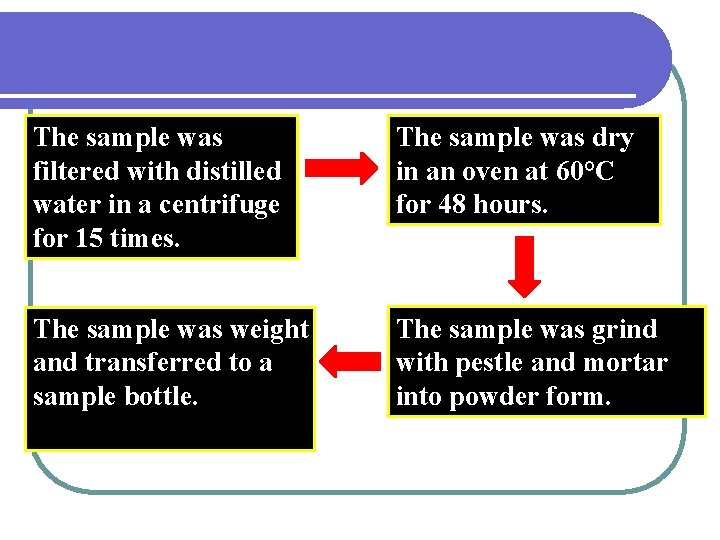
The sample was filtered with distilled water in a centrifuge for 15 times. The sample was dry in an oven at 60°C for 48 hours. The sample was weight and transferred to a sample bottle. The sample was grind with pestle and mortar into powder form.
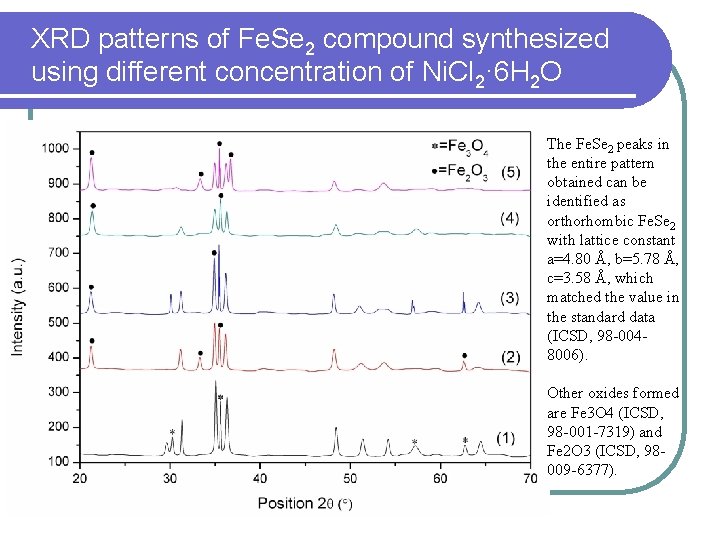
XRD patterns of Fe. Se 2 compound synthesized using different concentration of Ni. Cl 2· 6 H 2 O The Fe. Se 2 peaks in the entire pattern obtained can be identified as orthorhombic Fe. Se 2 with lattice constant a=4. 80 Å, b=5. 78 Å, c=3. 58 Å, which matched the value in the standard data (ICSD, 98 -0048006). Other oxides formed are Fe 3 O 4 (ICSD, 98 -001 -7319) and Fe 2 O 3 (ICSD, 98009 -6377).
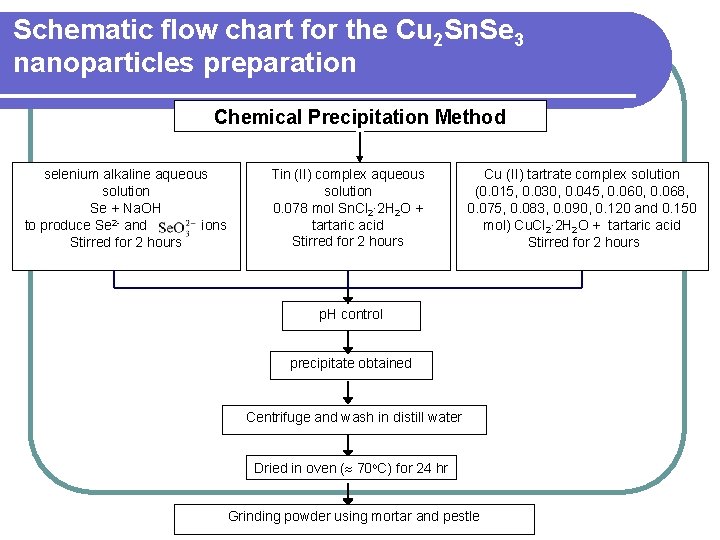
Schematic flow chart for the Cu 2 Sn. Se 3 nanoparticles preparation Chemical Precipitation Method selenium alkaline aqueous solution Se + Na. OH to produce Se 2 - and ions Stirred for 2 hours Tin (II) complex aqueous solution 0. 078 mol Sn. Cl 2∙ 2 H 2 O + tartaric acid Stirred for 2 hours Cu (II) tartrate complex solution (0. 015, 0. 030, 0. 045, 0. 060, 0. 068, 0. 075, 0. 083, 0. 090, 0. 120 and 0. 150 mol) Cu. Cl 2∙ 2 H 2 O + tartaric acid Stirred for 2 hours p. H control Mixture stir for 24 hours precipitate obtained Centrifuge and wash in distill water Dried in oven ( 70 o. C) for 24 hr Grinding powder using mortar and pestle
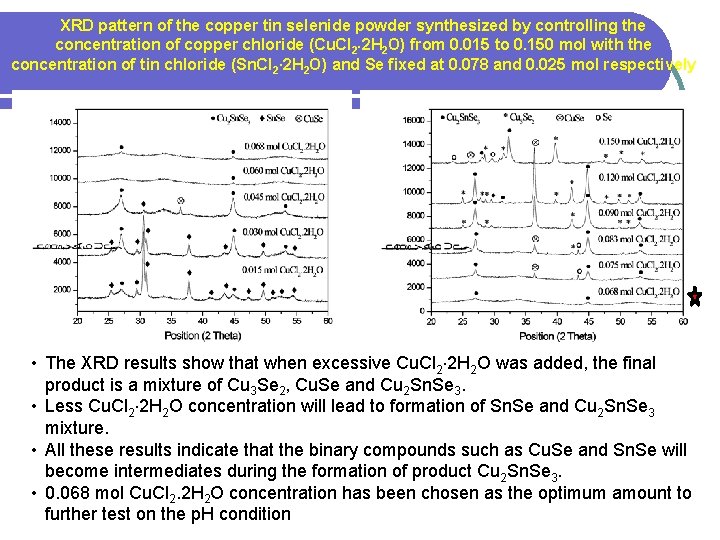
XRD pattern of the copper tin selenide powder synthesized by controlling the concentration of copper chloride (Cu. Cl 2 2 H 2 O) from 0. 015 to 0. 150 mol with the concentration of tin chloride (Sn. Cl 2∙ 2 H 2 O) and Se fixed at 0. 078 and 0. 025 mol respectively • The XRD results show that when excessive Cu. Cl 2 2 H 2 O was added, the final product is a mixture of Cu 3 Se 2, Cu. Se and Cu 2 Sn. Se 3. • Less Cu. Cl 2 2 H 2 O concentration will lead to formation of Sn. Se and Cu 2 Sn. Se 3 mixture. • All these results indicate that the binary compounds such as Cu. Se and Sn. Se will become intermediates during the formation of product Cu 2 Sn. Se 3. • 0. 068 mol Cu. Cl 2. 2 H 2 O concentration has been chosen as the optimum amount to further test on the p. H condition
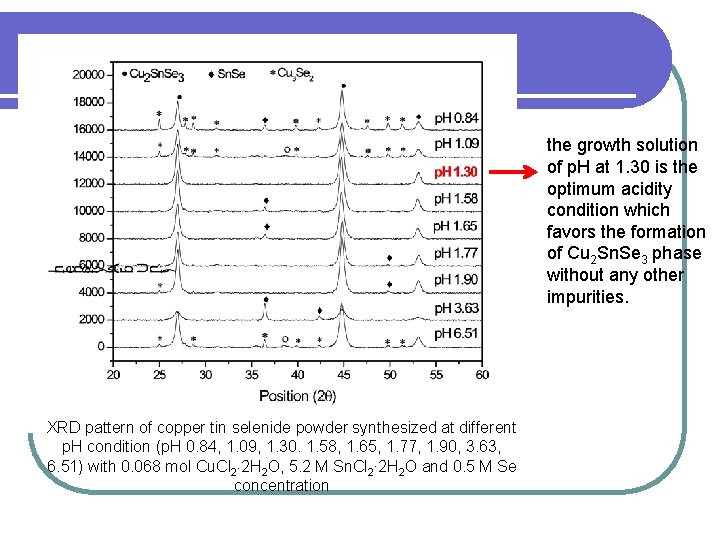
the growth solution of p. H at 1. 30 is the optimum acidity condition which favors the formation of Cu 2 Sn. Se 3 phase without any other impurities. XRD pattern of copper tin selenide powder synthesized at different p. H condition (p. H 0. 84, 1. 09, 1. 30. 1. 58, 1. 65, 1. 77, 1. 90, 3. 63, 6. 51) with 0. 068 mol Cu. Cl 2 2 H 2 O, 5. 2 M Sn. Cl 2∙ 2 H 2 O and 0. 5 M Se concentration
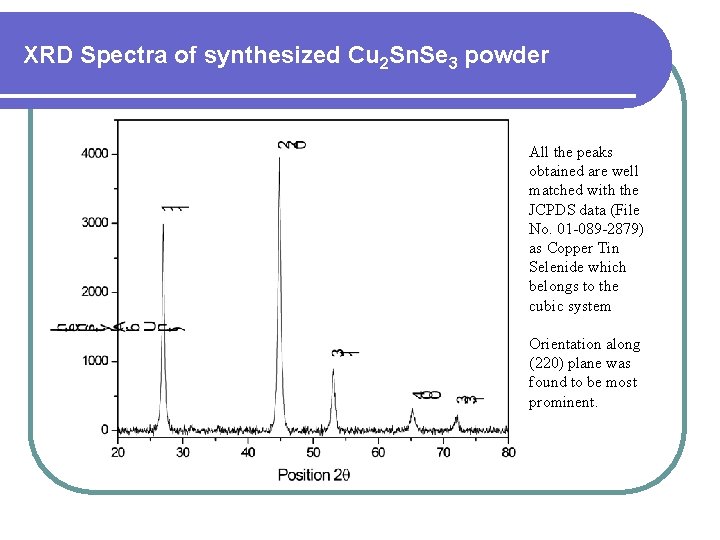
XRD Spectra of synthesized Cu 2 Sn. Se 3 powder All the peaks obtained are well matched with the JCPDS data (File No. 01 -089 -2879) as Copper Tin Selenide which belongs to the cubic system Orientation along (220) plane was found to be most prominent.
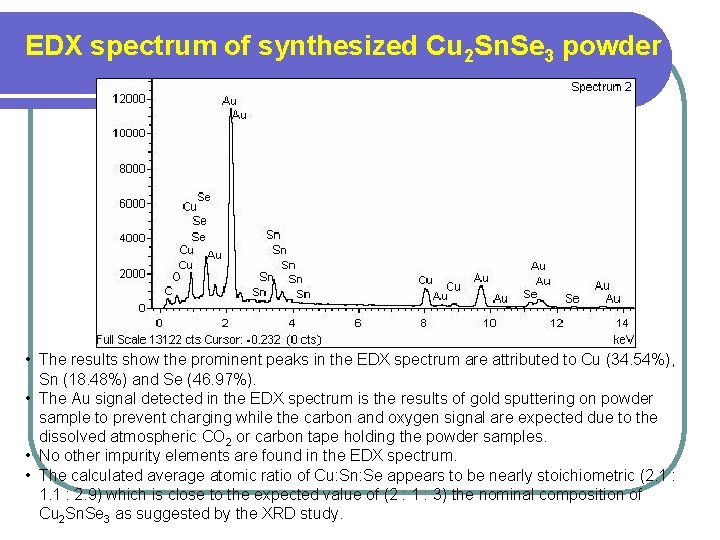
EDX spectrum of synthesized Cu 2 Sn. Se 3 powder • The results show the prominent peaks in the EDX spectrum are attributed to Cu (34. 54%), Sn (18. 48%) and Se (46. 97%). • The Au signal detected in the EDX spectrum is the results of gold sputtering on powder sample to prevent charging while the carbon and oxygen signal are expected due to the dissolved atmospheric CO 2 or carbon tape holding the powder samples. • No other impurity elements are found in the EDX spectrum. • The calculated average atomic ratio of Cu: Sn: Se appears to be nearly stoichiometric (2. 1 : 1. 1 : 2. 9) which is close to the expected value of (2 : 1 : 3) the nominal composition of Cu 2 Sn. Se 3 as suggested by the XRD study.
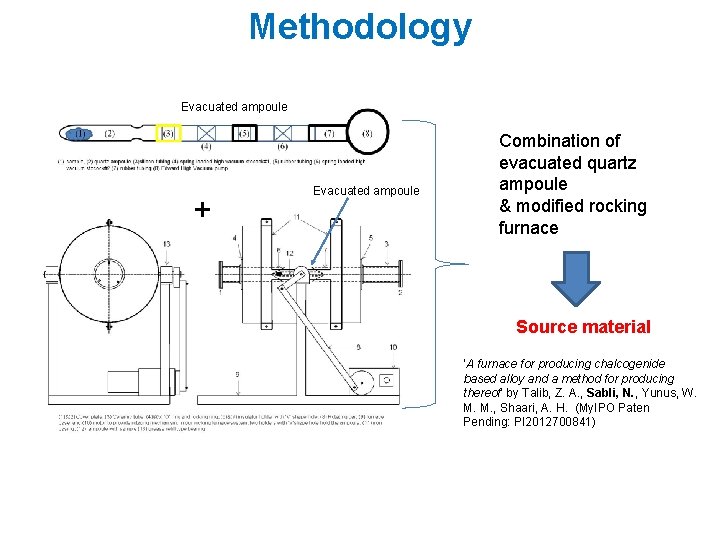
Methodology Evacuated ampoule + Evacuated ampoule Combination of evacuated quartz ampoule & modified rocking furnace Source material ‘A furnace for producing chalcogenide based alloy and a method for producing thereof’ by Talib, Z. A. , Sabli, N. , Yunus, W. M. M. , Shaari, A. H. (My. IPO Paten Pending: PI 2012700841)
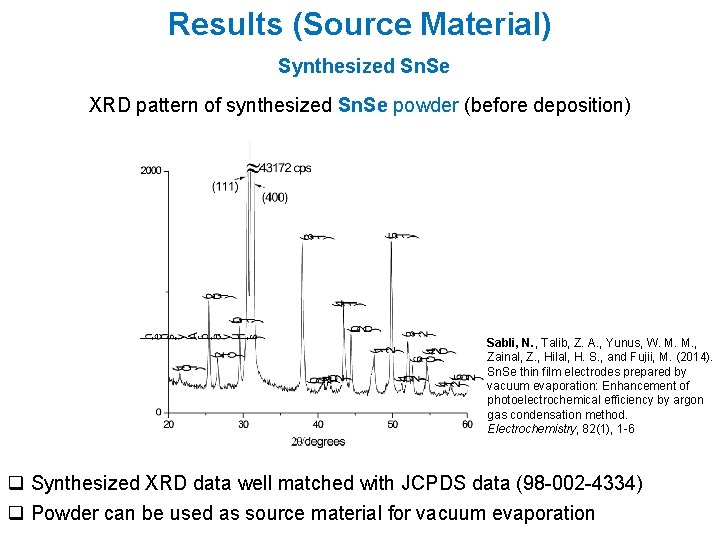
Results (Source Material) Synthesized Sn. Se XRD pattern of synthesized Sn. Se powder (before deposition) Sabli, N. , Talib, Z. A. , Yunus, W. M. M. , Zainal, Z. , Hilal, H. S. , and Fujii, M. (2014). Sn. Se thin film electrodes prepared by vacuum evaporation: Enhancement of photoelectrochemical efficiency by argon gas condensation method. Electrochemistry, 82(1), 1 -6 q Synthesized XRD data well matched with JCPDS data (98 -002 -4334) q Powder can be used as source material for vacuum evaporation
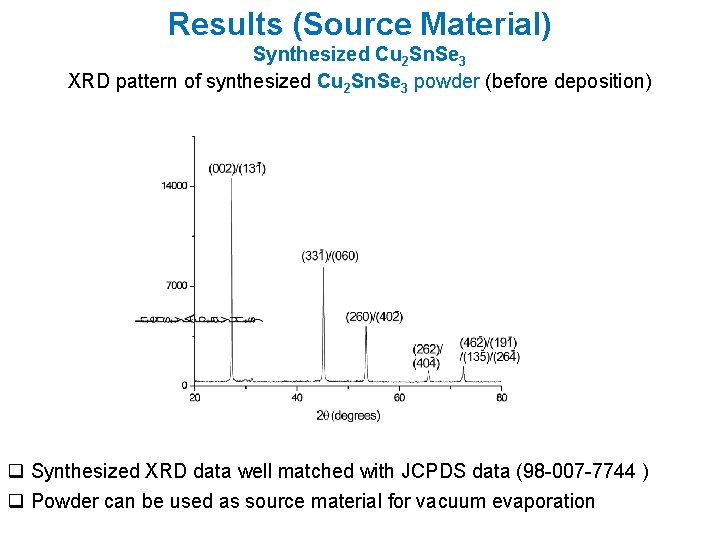
Results (Source Material) Synthesized Cu 2 Sn. Se 3 XRD pattern of synthesized Cu 2 Sn. Se 3 powder (before deposition) q Synthesized XRD data well matched with JCPDS data (98 -007 -7744 ) q Powder can be used as source material for vacuum evaporation
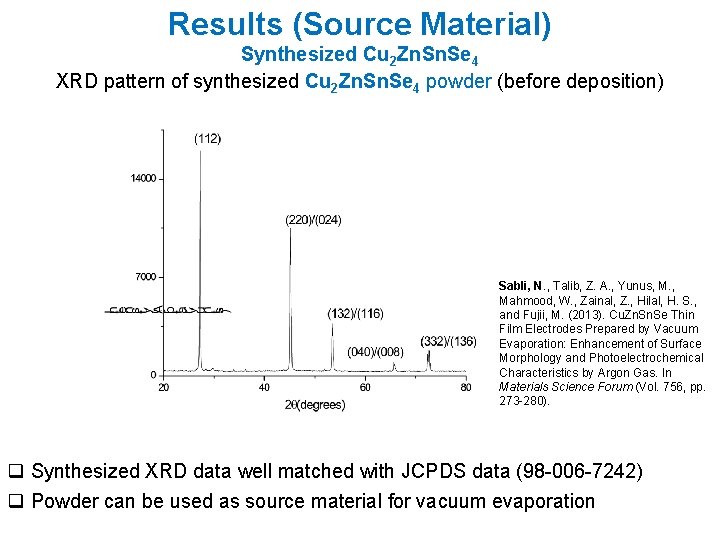
Results (Source Material) Synthesized Cu 2 Zn. Se 4 XRD pattern of synthesized Cu 2 Zn. Se 4 powder (before deposition) Sabli, N. , Talib, Z. A. , Yunus, M. , Mahmood, W. , Zainal, Z. , Hilal, H. S. , and Fujii, M. (2013). Cu. Zn. Se Thin Film Electrodes Prepared by Vacuum Evaporation: Enhancement of Surface Morphology and Photoelectrochemical Characteristics by Argon Gas. In Materials Science Forum (Vol. 756, pp. 273 -280). q Synthesized XRD data well matched with JCPDS data (98 -006 -7242) q Powder can be used as source material for vacuum evaporation

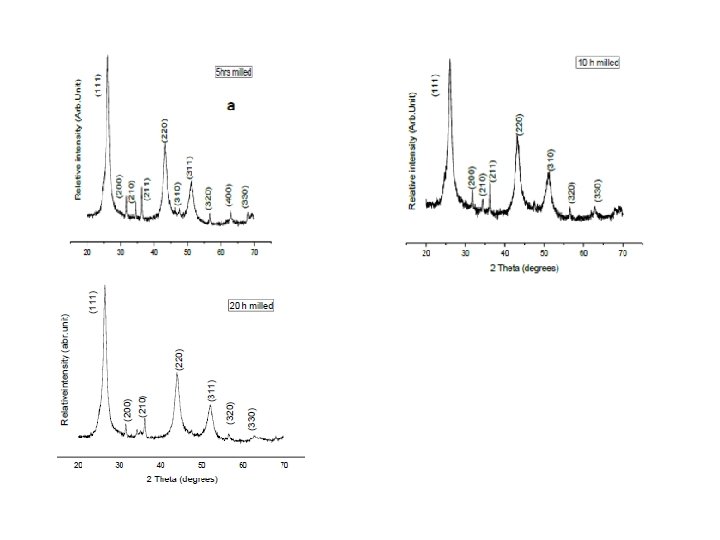
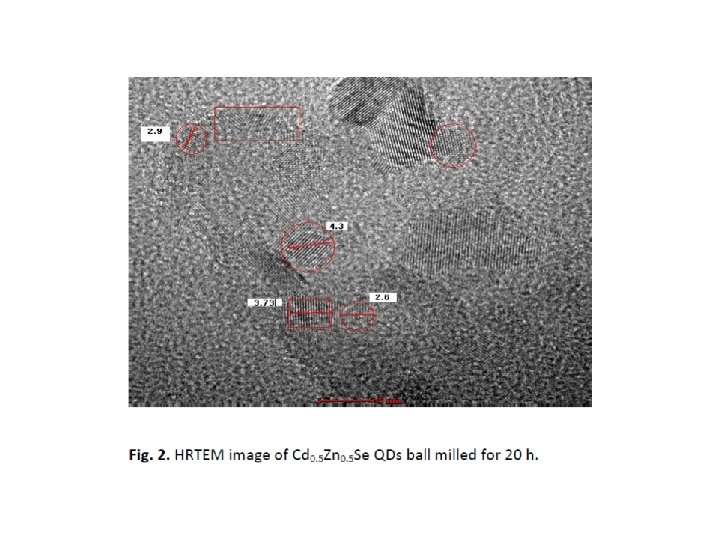
Mechanochemical solid state synthesis of Cd 0. 5 Zn 0. 5 Se The starting materials were high-purity cadmium (99. 99%), zinc (99. 99%) and selenium (99. 99%) elemental powders purchased from Alfa Aesar. Mixtures at the desired atomic ratios were placed in a stainless steel grinding jar with stainless balls under an inert atmosphere. The intensive grinding the mixtures was performed in a high-energy planetary ball mill PM 100 (Retsch) with a ball-to-powder ratio of 10: 1. Grinding balls of 3 mm in diameter were used. The milling time was varied from 5 to 20 hours at a speed of 500 rpm. Small quantities of the as-milled powders were removed from the grinding jar at various time intervals for microstructural and optical characterization.

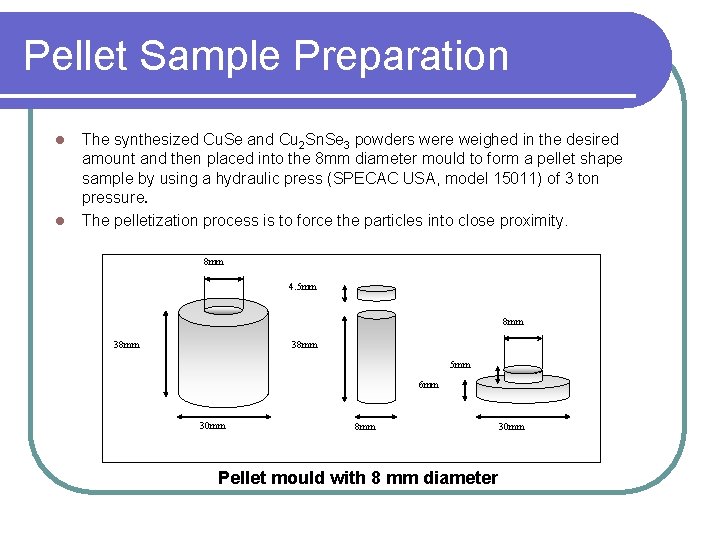
Pellet Sample Preparation l l The synthesized Cu. Se and Cu 2 Sn. Se 3 powders were weighed in the desired amount and then placed into the 8 mm diameter mould to form a pellet shape sample by using a hydraulic press (SPECAC USA, model 15011) of 3 ton pressure. The pelletization process is to force the particles into close proximity. 8 mm 4. 5 mm 8 mm 38 mm 5 mm 6 mm 30 mm 8 mm Pellet mould with 8 mm diameter 30 mm
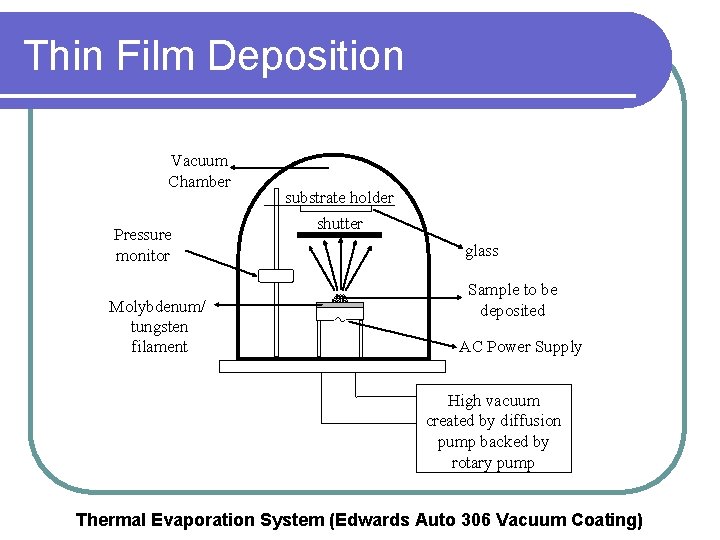
Thin Film Deposition Vacuum Chamber Pressure monitor Molybdenum/ tungsten filament substrate holder shutter glass Sample to be deposited AC Power Supply High vacuum created by diffusion pump backed by rotary pump Thermal Evaporation System (Edwards Auto 306 Vacuum Coating)
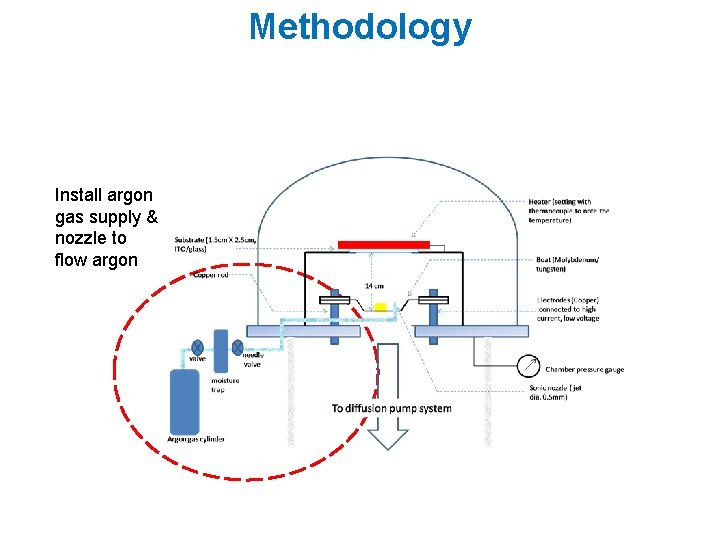
Methodology Install argon gas supply & nozzle to flow argon
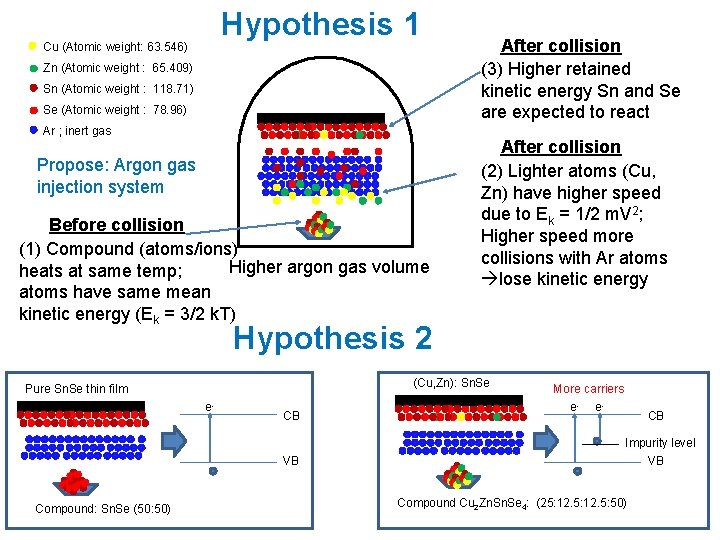
Hypothesis 1 Cu (Atomic weight: 63. 546) Zn (Atomic weight : 65. 409) Sn (Atomic weight : 118. 71) Se (Atomic weight : 78. 96) Ar ; inert gas Propose: Argon gas injection system Before collision (1) Compound (atoms/ions) Higher argon gas volume heats at same temp; atoms have same mean kinetic energy (Ek = 3/2 k. T) After collision (3) Higher retained kinetic energy Sn and Se are expected to react After collision (2) Lighter atoms (Cu, Zn) have higher speed due to Ek = 1/2 m. V 2; Higher speed more collisions with Ar atoms lose kinetic energy Hypothesis 2 (Cu, Zn): Sn. Se Pure Sn. Se thin film e- CB VB Compound: Sn. Se (50: 50) More carriers e- e- CB Impurity level VB Compound Cu 2 Zn. Se 4: (25: 12. 5: 50)
Annealing process For the heat treatment process, the Cu. Se and Cu 2 Sn. Se 3 film were placed on the quartz boat and heated with gas N 2 (1 cc/min) by using furnace. l The annealing process was carried out at a temperature raised from room temperature 26 o. C to 100 o. C, 200 o. C, 300 o. C, 400 o. C at an increasing rate (2 o. C/min). l Upon reaching the required temperature, it was maintained for 3 hours. l The temperature was then natural cooling to room temperature for 24 hours. l
Methodology
XRD (Pan. Analytical X’pert PRO PW 3040)
FESEM, EDX and TEM Field Emission Scanning Electron Microscope (JOEL JSM-6700 F) Transmission Electron Microscope (Hitachi H 7100 TEM) Energy Dispersive X-Ray (EDX) (LEO 1455 VP SEM )
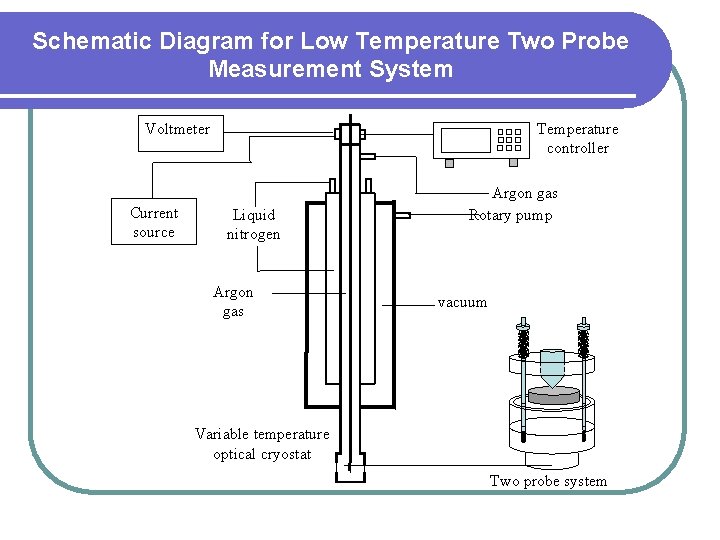
Schematic Diagram for Low Temperature Two Probe Measurement System Voltmeter Current source Temperature controller Liquid nitrogen Argon gas Rotary pump vacuum Variable temperature optical cryostat Two probe system
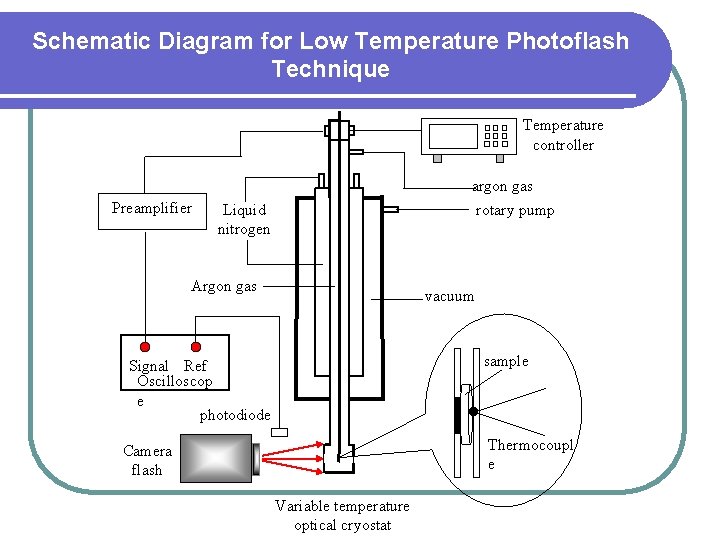
Schematic Diagram for Low Temperature Photoflash Technique Temperature controller argon gas Preamplifier Liquid nitrogen rotary pump Argon gas vacuum sample Signal Ref Oscilloscop e photodiode Thermocoupl e Camera flash Variable temperature optical cryostat
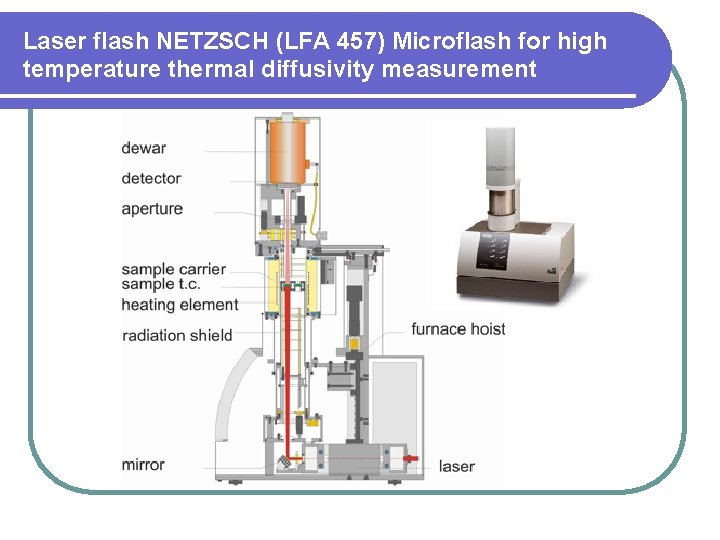
Laser flash NETZSCH (LFA 457) Microflash for high temperature thermal diffusivity measurement
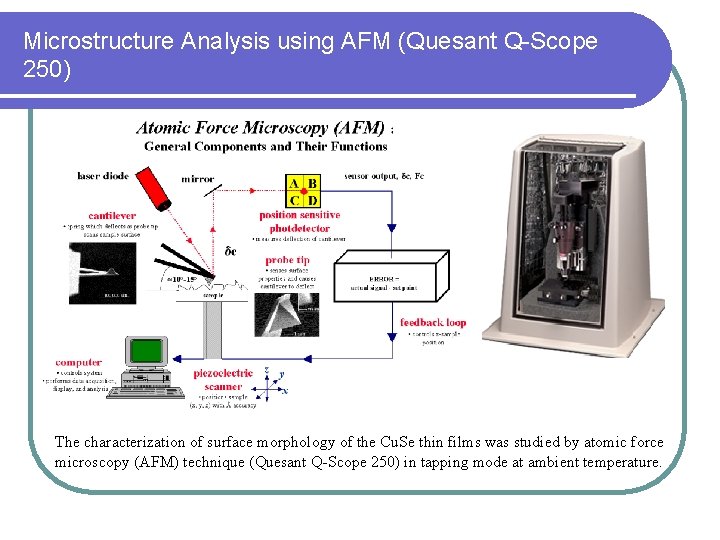
Microstructure Analysis using AFM (Quesant Q-Scope 250) The characterization of surface morphology of the Cu. Se thin films was studied by atomic force microscopy (AFM) technique (Quesant Q-Scope 250) in tapping mode at ambient temperature.
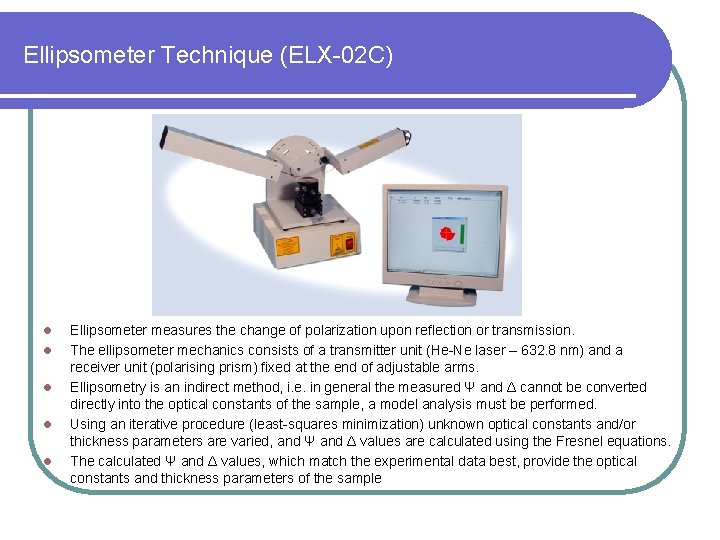
Ellipsometer Technique (ELX-02 C) l l l Ellipsometer measures the change of polarization upon reflection or transmission. The ellipsometer mechanics consists of a transmitter unit (He-Ne laser – 632. 8 nm) and a receiver unit (polarising prism) fixed at the end of adjustable arms. Ellipsometry is an indirect method, i. e. in general the measured Ψ and Δ cannot be converted directly into the optical constants of the sample, a model analysis must be performed. Using an iterative procedure (least-squares minimization) unknown optical constants and/or thickness parameters are varied, and Ψ and Δ values are calculated using the Fresnel equations. The calculated Ψ and Δ values, which match the experimental data best, provide the optical constants and thickness parameters of the sample
Fiber Optic Spectrophotometer ØThe optical studies of Cu. Se film analyzed using Ocean Fiber Optics Spectrophotometer. ØThe transmittance spectra in the region 300 nnm – 800 nm has been collected and optical parameters such as optical absorption coefficient and optical band gap has been evaluated.
Structural Analysis X-Ray Powder Diffraction
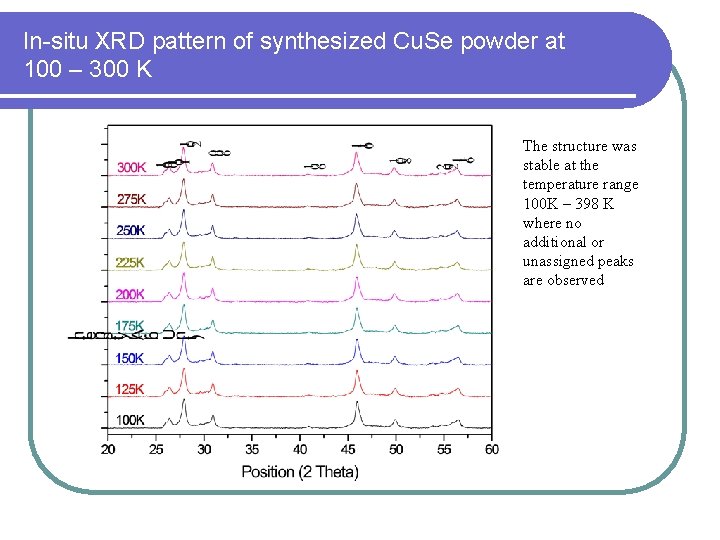
In-situ XRD pattern of synthesized Cu. Se powder at 100 – 300 K The structure was stable at the temperature range 100 K – 398 K where no additional or unassigned peaks are observed
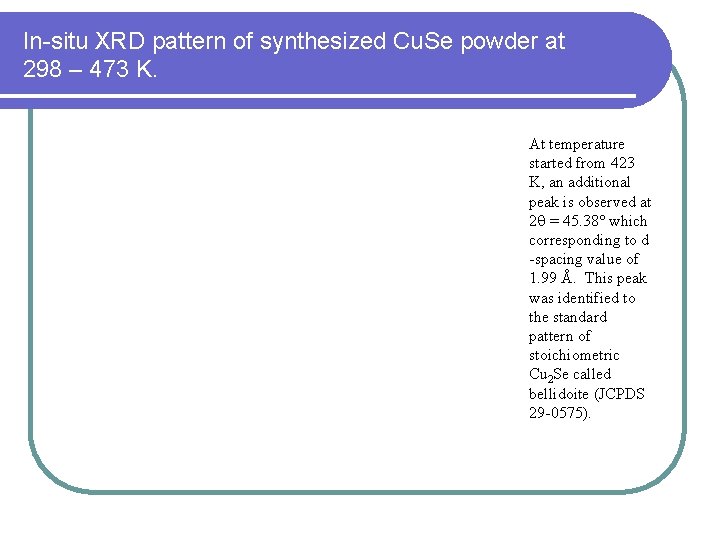
In-situ XRD pattern of synthesized Cu. Se powder at 298 – 473 K. At temperature started from 423 K, an additional peak is observed at 2 = 45. 38 which corresponding to d -spacing value of 1. 99 Å. This peak was identified to the standard pattern of stoichiometric Cu 2 Se called bellidoite (JCPDS 29 -0575).
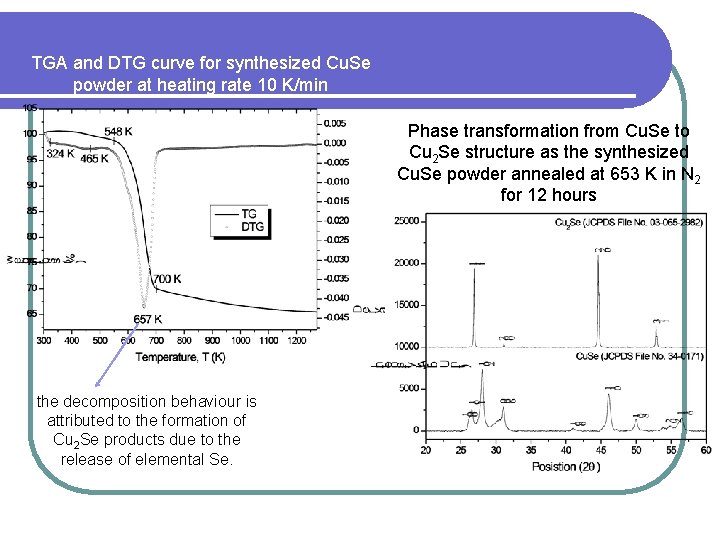
TGA and DTG curve for synthesized Cu. Se powder at heating rate 10 K/min Phase transformation from Cu. Se to Cu 2 Se structure as the synthesized Cu. Se powder annealed at 653 K in N 2 for 12 hours the decomposition behaviour is attributed to the formation of Cu 2 Se products due to the release of elemental Se.
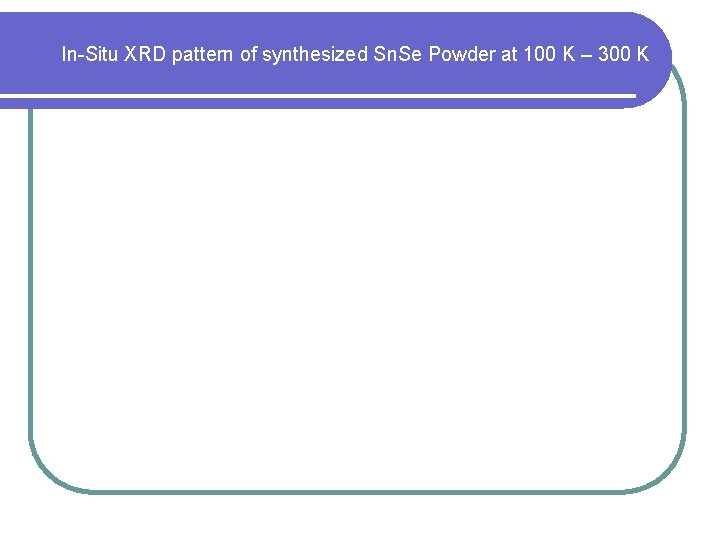
In-Situ XRD pattern of synthesized Sn. Se Powder at 100 K – 300 K
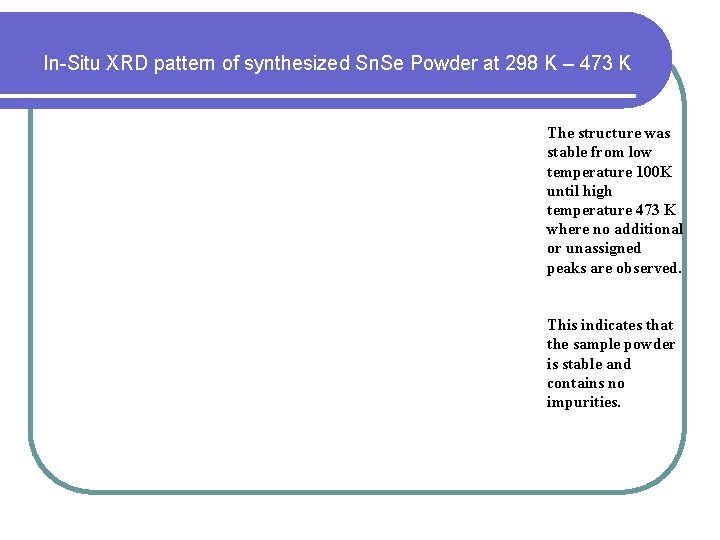
In-Situ XRD pattern of synthesized Sn. Se Powder at 298 K – 473 K The structure was stable from low temperature 100 K until high temperature 473 K where no additional or unassigned peaks are observed. This indicates that the sample powder is stable and contains no impurities.

Annealing at 1173 K destroys the Sn. Se lattice (peaks of Sn. Se disappear in the sample) and leads to formation of Sn. O 2 and Sn phases in the presence of oxygen and release of free selenium followed the reaction in eq. (5. 9) [56, 57]: 2 Sn. Se + 3 O 2 = Sn. O 2 + Sn + 2 Se. O 2
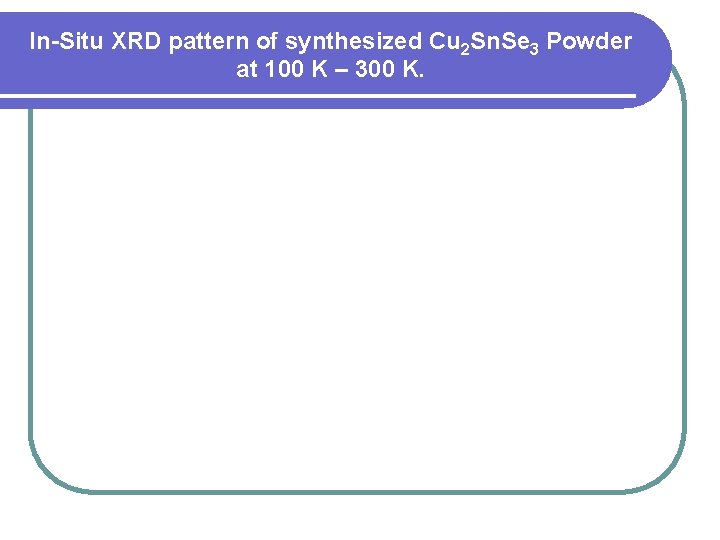
In-Situ XRD pattern of synthesized Cu 2 Sn. Se 3 Powder at 100 K – 300 K.
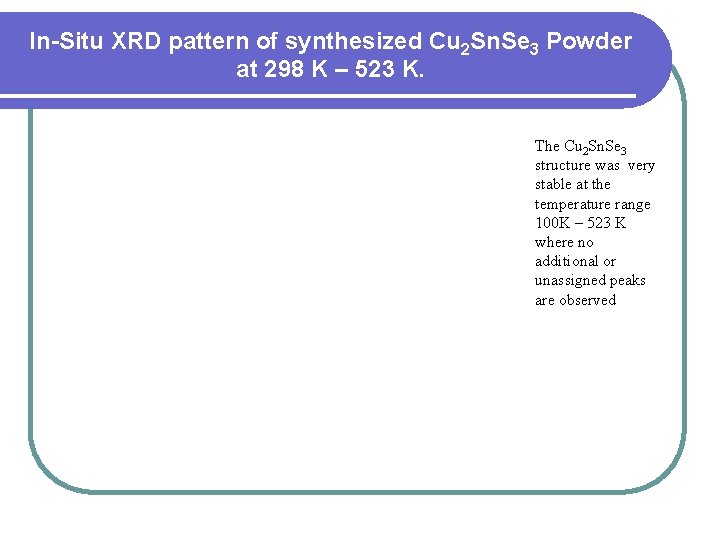
In-Situ XRD pattern of synthesized Cu 2 Sn. Se 3 Powder at 298 K – 523 K. The Cu 2 Sn. Se 3 structure was very stable at the temperature range 100 K – 523 K where no additional or unassigned peaks are observed
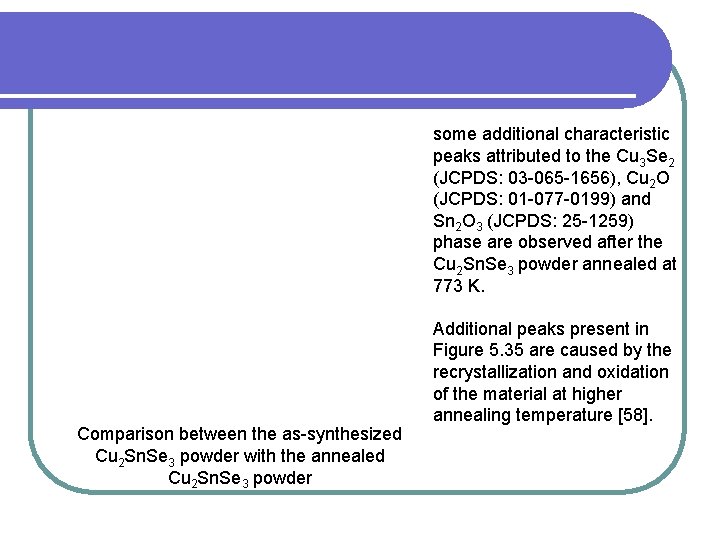
some additional characteristic peaks attributed to the Cu 3 Se 2 (JCPDS: 03 -065 -1656), Cu 2 O (JCPDS: 01 -077 -0199) and Sn 2 O 3 (JCPDS: 25 -1259) phase are observed after the Cu 2 Sn. Se 3 powder annealed at 773 K. Comparison between the as-synthesized Cu 2 Sn. Se 3 powder with the annealed Cu 2 Sn. Se 3 powder Additional peaks present in Figure 5. 35 are caused by the recrystallization and oxidation of the material at higher annealing temperature [58].

Morphology
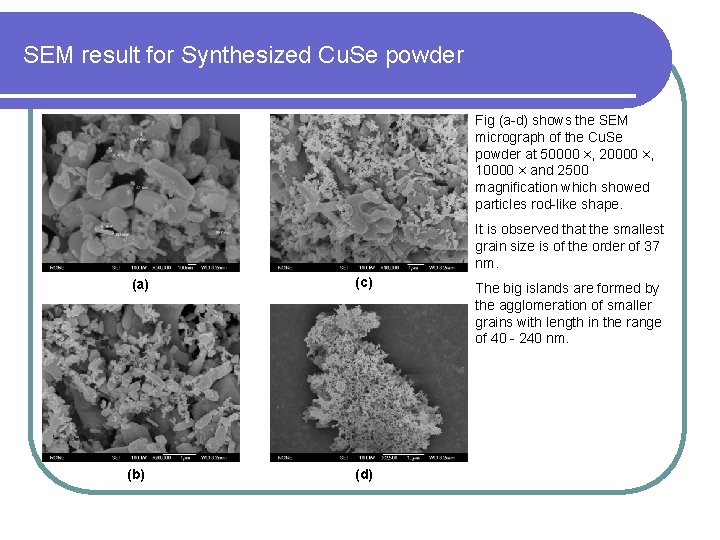
SEM result for Synthesized Cu. Se powder Fig (a-d) shows the SEM micrograph of the Cu. Se powder at 50000 ×, 20000 ×, 10000 × and 2500 magnification which showed particles rod-like shape. It is observed that the smallest grain size is of the order of 37 nm. (a) (c) (b) (d) The big islands are formed by the agglomeration of smaller grains with length in the range of 40 - 240 nm.
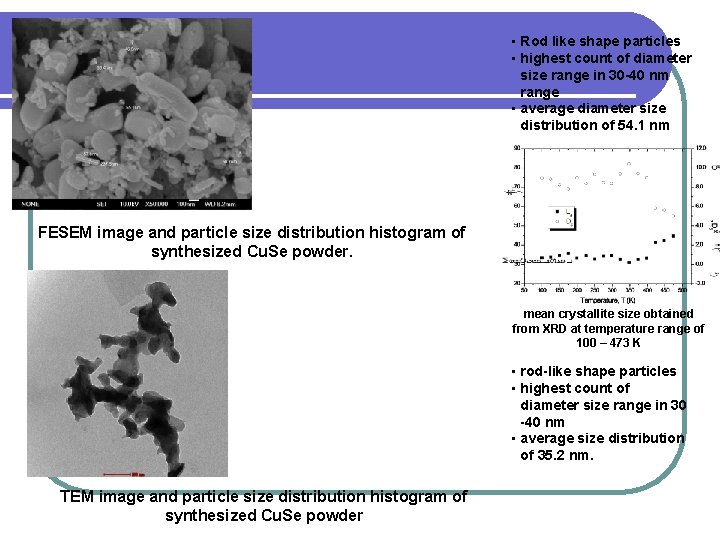
• Rod like shape particles • highest count of diameter size range in 30 -40 nm range • average diameter size distribution of 54. 1 nm FESEM image and particle size distribution histogram of synthesized Cu. Se powder. mean crystallite size obtained from XRD at temperature range of 100 – 473 K • rod-like shape particles • highest count of diameter size range in 30 -40 nm • average size distribution of 35. 2 nm. TEM image and particle size distribution histogram of synthesized Cu. Se powder
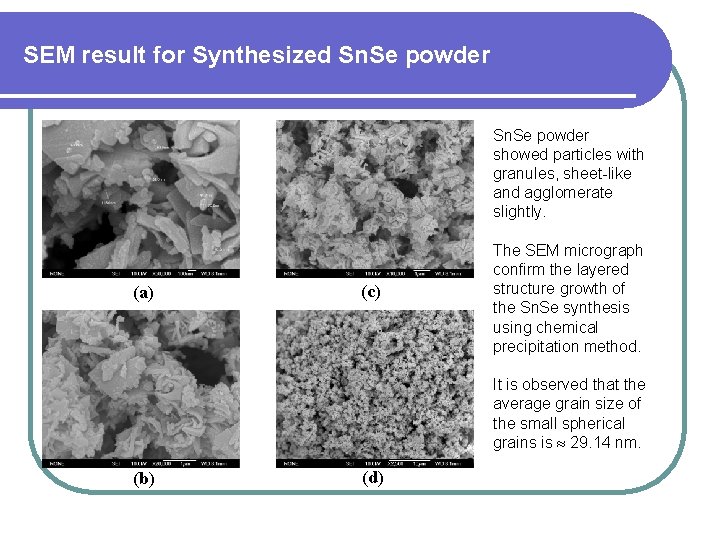
SEM result for Synthesized Sn. Se powder showed particles with granules, sheet-like and agglomerate slightly. (a) (c) The SEM micrograph confirm the layered structure growth of the Sn. Se synthesis using chemical precipitation method. It is observed that the average grain size of the small spherical grains is 29. 14 nm. (b) (d)
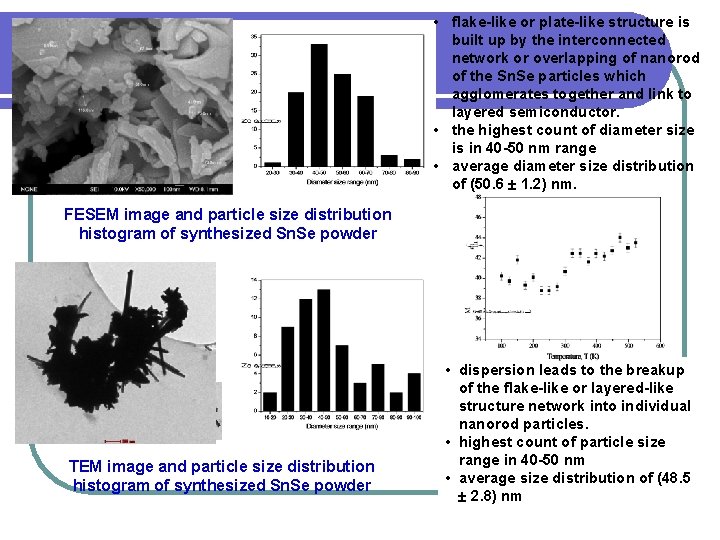
• flake-like or plate-like structure is built up by the interconnected network or overlapping of nanorod of the Sn. Se particles which agglomerates together and link to layered semiconductor. • the highest count of diameter size is in 40 -50 nm range • average diameter size distribution of (50. 6 1. 2) nm. FESEM image and particle size distribution histogram of synthesized Sn. Se powder TEM image and particle size distribution histogram of synthesized Sn. Se powder • dispersion leads to the breakup of the flake-like or layered-like structure network into individual nanorod particles. • highest count of particle size range in 40 -50 nm • average size distribution of (48. 5 2. 8) nm
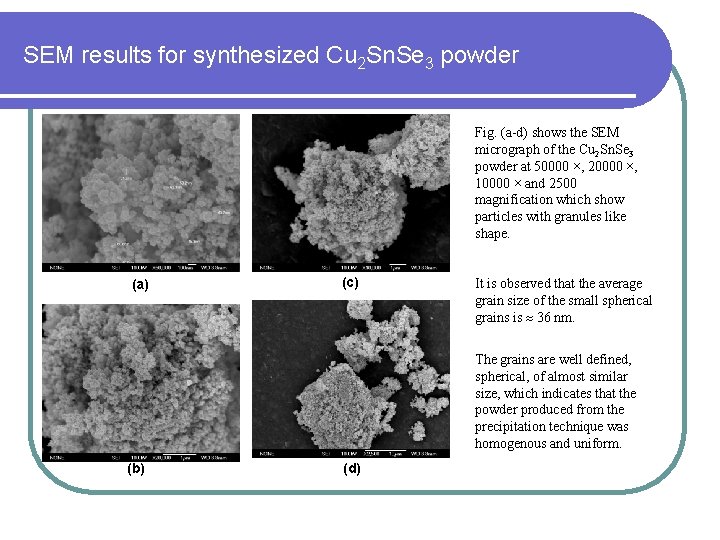
SEM results for synthesized Cu 2 Sn. Se 3 powder Fig. (a-d) shows the SEM micrograph of the Cu 2 Sn. Se 3 powder at 50000 ×, 20000 ×, 10000 × and 2500 magnification which show particles with granules like shape. (a) (c) It is observed that the average grain size of the small spherical grains is 36 nm. The grains are well defined, spherical, of almost similar size, which indicates that the powder produced from the precipitation technique was homogenous and uniform. (b) (d)
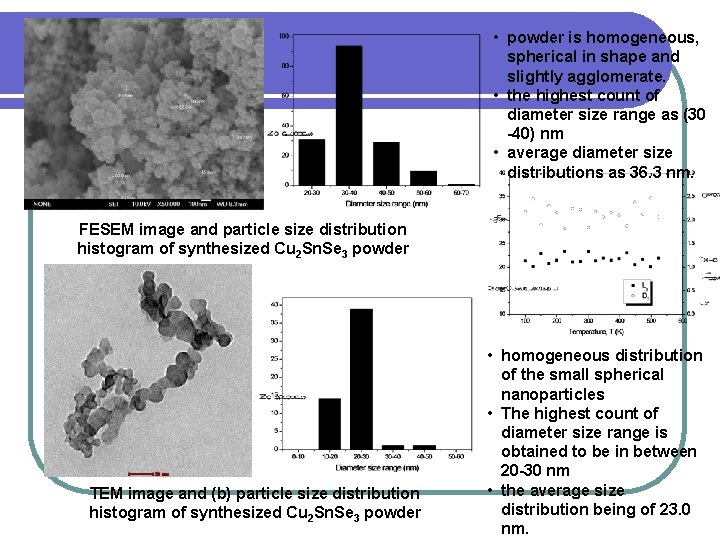
• powder is homogeneous, spherical in shape and slightly agglomerate. • the highest count of diameter size range as (30 -40) nm • average diameter size distributions as 36. 3 nm. FESEM image and particle size distribution histogram of synthesized Cu 2 Sn. Se 3 powder TEM image and (b) particle size distribution histogram of synthesized Cu 2 Sn. Se 3 powder • homogeneous distribution of the small spherical nanoparticles • The highest count of diameter size range is obtained to be in between 20 -30 nm • the average size distribution being of 23. 0 nm.
Electrical Properties
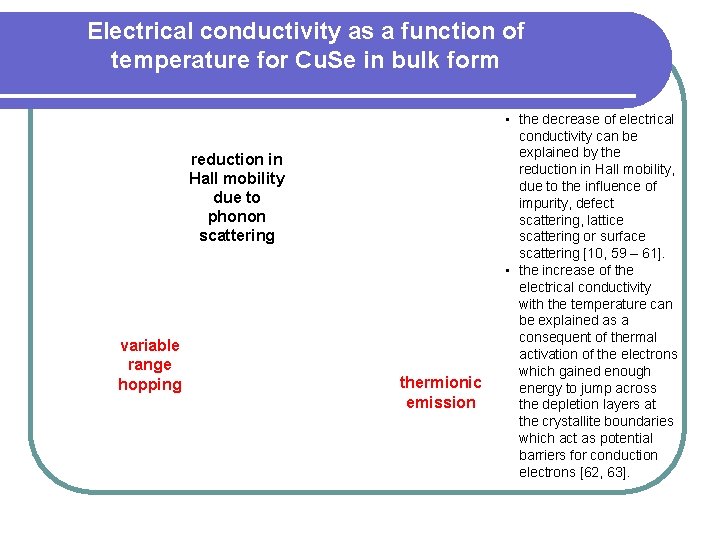
Electrical conductivity as a function of temperature for Cu. Se in bulk form reduction in Hall mobility due to phonon scattering variable range hopping thermionic emission • the decrease of electrical conductivity can be explained by the reduction in Hall mobility, due to the influence of impurity, defect scattering, lattice scattering or surface scattering [10, 59 – 61]. • the increase of the electrical conductivity with the temperature can be explained as a consequent of thermal activation of the electrons which gained enough energy to jump across the depletion layers at the crystallite boundaries which act as potential barriers for conduction electrons [62, 63].
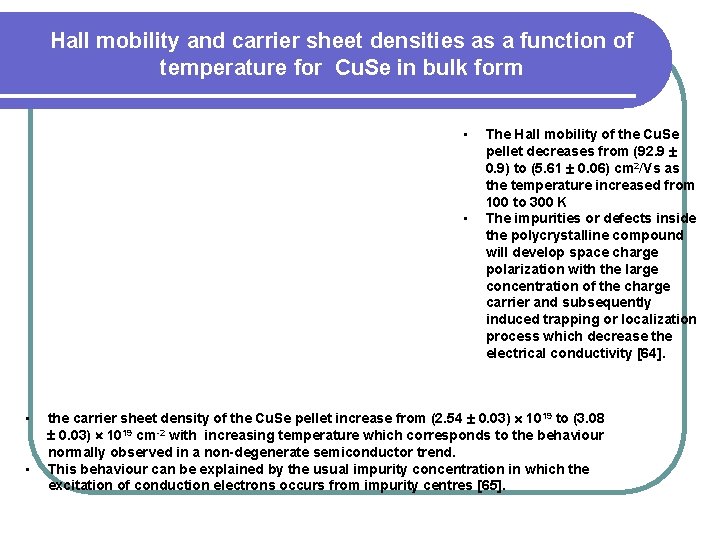
Hall mobility and carrier sheet densities as a function of temperature for Cu. Se in bulk form • • The Hall mobility of the Cu. Se pellet decreases from (92. 9 0. 9) to (5. 61 0. 06) cm 2/Vs as the temperature increased from 100 to 300 K The impurities or defects inside the polycrystalline compound will develop space charge polarization with the large concentration of the charge carrier and subsequently induced trapping or localization process which decrease the electrical conductivity [64]. the carrier sheet density of the Cu. Se pellet increase from (2. 54 0. 03) 1019 to (3. 08 0. 03) 1019 cm-2 with increasing temperature which corresponds to the behaviour normally observed in a non-degenerate semiconductor trend. This behaviour can be explained by the usual impurity concentration in which the excitation of conduction electrons occurs from impurity centres [65].
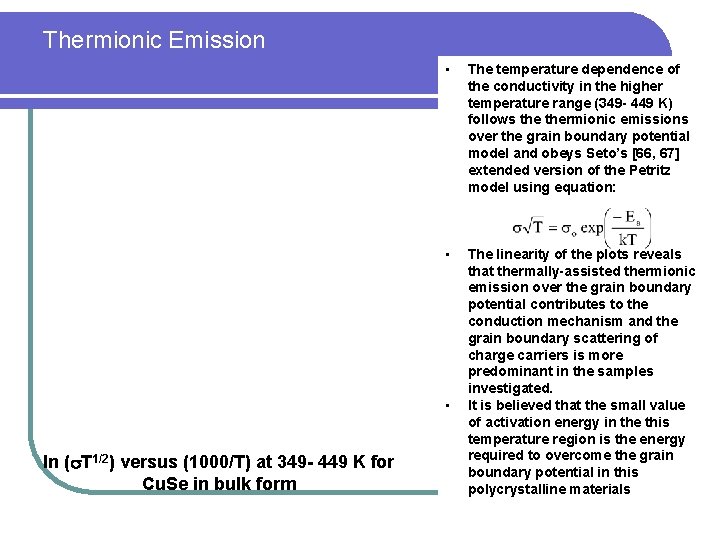
Thermionic Emission • The temperature dependence of the conductivity in the higher temperature range (349 - 449 K) follows thermionic emissions over the grain boundary potential model and obeys Seto’s [66, 67] extended version of the Petritz model using equation: • The linearity of the plots reveals that thermally-assisted thermionic emission over the grain boundary potential contributes to the conduction mechanism and the grain boundary scattering of charge carriers is more predominant in the samples investigated. It is believed that the small value of activation energy in the this temperature region is the energy required to overcome the grain boundary potential in this polycrystalline materials • ln ( T 1/2) versus (1000/T) at 349 - 449 K for Cu. Se in bulk form
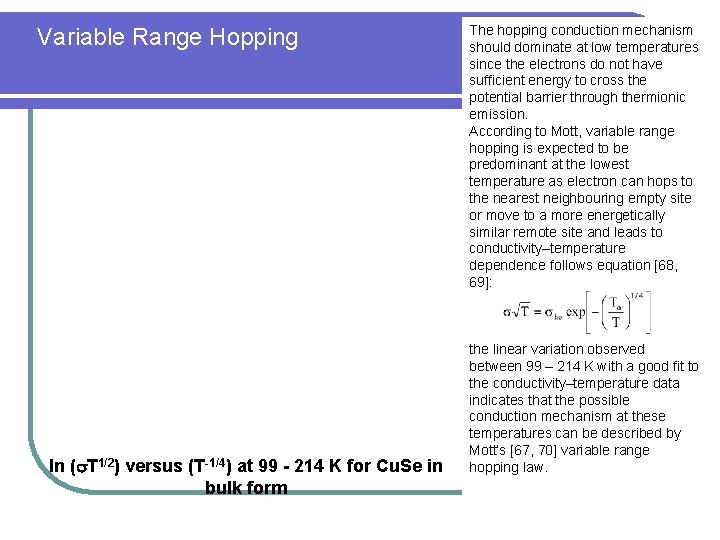
Variable Range Hopping ln ( T 1/2) versus (T-1/4) at 99 - 214 K for Cu. Se in bulk form The hopping conduction mechanism should dominate at low temperatures since the electrons do not have sufficient energy to cross the potential barrier through thermionic emission. According to Mott, variable range hopping is expected to be predominant at the lowest temperature as electron can hops to the nearest neighbouring empty site or move to a more energetically similar remote site and leads to conductivity–temperature dependence follows equation [68, 69]: the linear variation observed between 99 – 214 K with a good fit to the conductivity–temperature data indicates that the possible conduction mechanism at these temperatures can be described by Mott’s [67, 70] variable range hopping law.
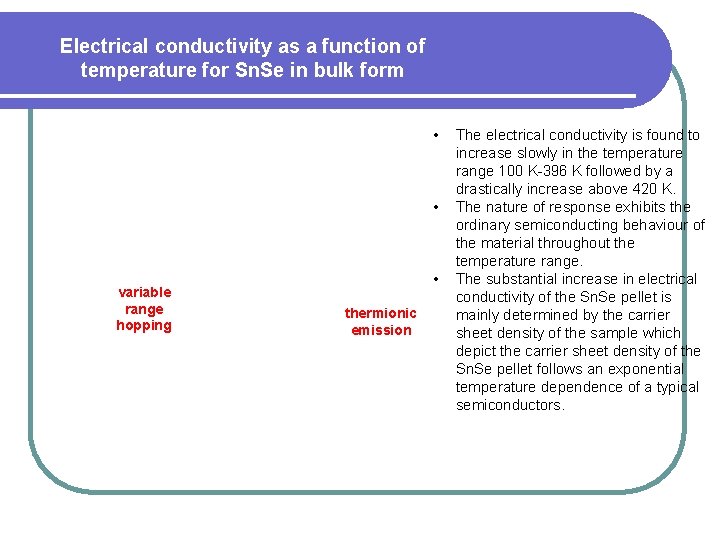
Electrical conductivity as a function of temperature for Sn. Se in bulk form • • variable range hopping • thermionic emission The electrical conductivity is found to increase slowly in the temperature range 100 K-396 K followed by a drastically increase above 420 K. The nature of response exhibits the ordinary semiconducting behaviour of the material throughout the temperature range. The substantial increase in electrical conductivity of the Sn. Se pellet is mainly determined by the carrier sheet density of the sample which depict the carrier sheet density of the Sn. Se pellet follows an exponential temperature dependence of a typical semiconductors.
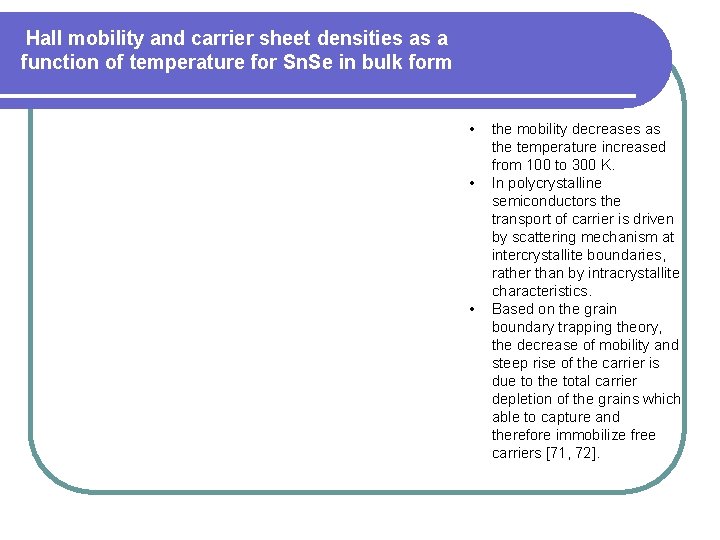
Hall mobility and carrier sheet densities as a function of temperature for Sn. Se in bulk form • • • the mobility decreases as the temperature increased from 100 to 300 K. In polycrystalline semiconductors the transport of carrier is driven by scattering mechanism at intercrystallite boundaries, rather than by intracrystallite characteristics. Based on the grain boundary trapping theory, the decrease of mobility and steep rise of the carrier is due to the total carrier depletion of the grains which able to capture and therefore immobilize free carriers [71, 72].
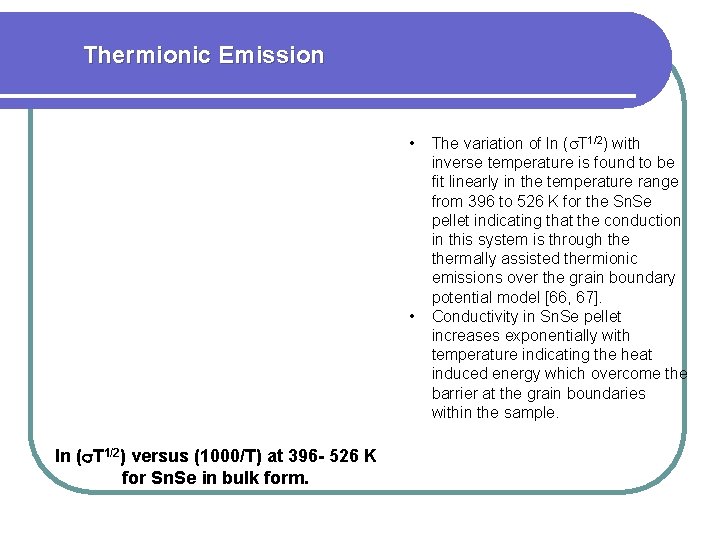
Thermionic Emission • • ln ( T 1/2) versus (1000/T) at 396 - 526 K for Sn. Se in bulk form. The variation of ln ( T 1/2) with inverse temperature is found to be fit linearly in the temperature range from 396 to 526 K for the Sn. Se pellet indicating that the conduction in this system is through thermally assisted thermionic emissions over the grain boundary potential model [66, 67]. Conductivity in Sn. Se pellet increases exponentially with temperature indicating the heat induced energy which overcome the barrier at the grain boundaries within the sample.
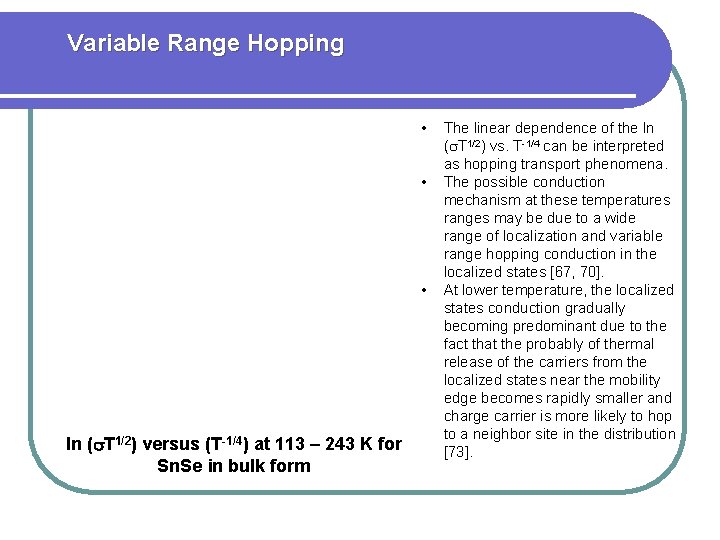
Variable Range Hopping • • • ln ( T 1/2) versus (T-1/4) at 113 – 243 K for Sn. Se in bulk form The linear dependence of the ln ( T 1/2) vs. T-1/4 can be interpreted as hopping transport phenomena. The possible conduction mechanism at these temperatures ranges may be due to a wide range of localization and variable range hopping conduction in the localized states [67, 70]. At lower temperature, the localized states conduction gradually becoming predominant due to the fact that the probably of thermal release of the carriers from the localized states near the mobility edge becomes rapidly smaller and charge carrier is more likely to hop to a neighbor site in the distribution [73].
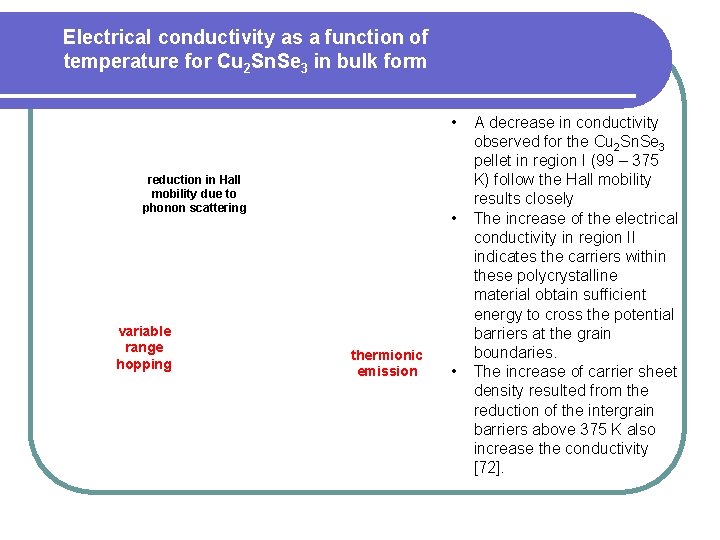
Electrical conductivity as a function of temperature for Cu 2 Sn. Se 3 in bulk form • reduction in Hall mobility due to phonon scattering variable range hopping • thermionic emission • A decrease in conductivity observed for the Cu 2 Sn. Se 3 pellet in region I (99 – 375 K) follow the Hall mobility results closely The increase of the electrical conductivity in region II indicates the carriers within these polycrystalline material obtain sufficient energy to cross the potential barriers at the grain boundaries. The increase of carrier sheet density resulted from the reduction of the intergrain barriers above 375 K also increase the conductivity [72].
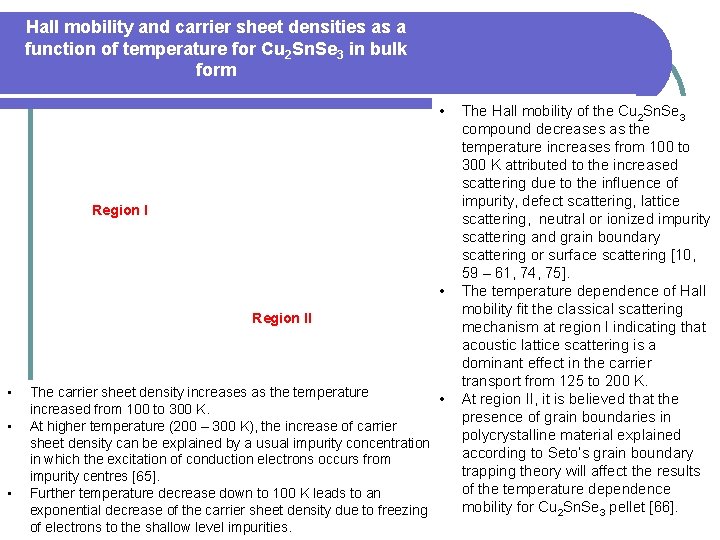
Hall mobility and carrier sheet densities as a function of temperature for Cu 2 Sn. Se 3 in bulk form • Region I • Region II • • • The carrier sheet density increases as the temperature • increased from 100 to 300 K. At higher temperature (200 – 300 K), the increase of carrier sheet density can be explained by a usual impurity concentration in which the excitation of conduction electrons occurs from impurity centres [65]. Further temperature decrease down to 100 K leads to an exponential decrease of the carrier sheet density due to freezing of electrons to the shallow level impurities. The Hall mobility of the Cu 2 Sn. Se 3 compound decreases as the temperature increases from 100 to 300 K attributed to the increased scattering due to the influence of impurity, defect scattering, lattice scattering, neutral or ionized impurity scattering and grain boundary scattering or surface scattering [10, 59 – 61, 74, 75]. The temperature dependence of Hall mobility fit the classical scattering mechanism at region I indicating that acoustic lattice scattering is a dominant effect in the carrier transport from 125 to 200 K. At region II, it is believed that the presence of grain boundaries in polycrystalline material explained according to Seto’s grain boundary trapping theory will affect the results of the temperature dependence mobility for Cu 2 Sn. Se 3 pellet [66].
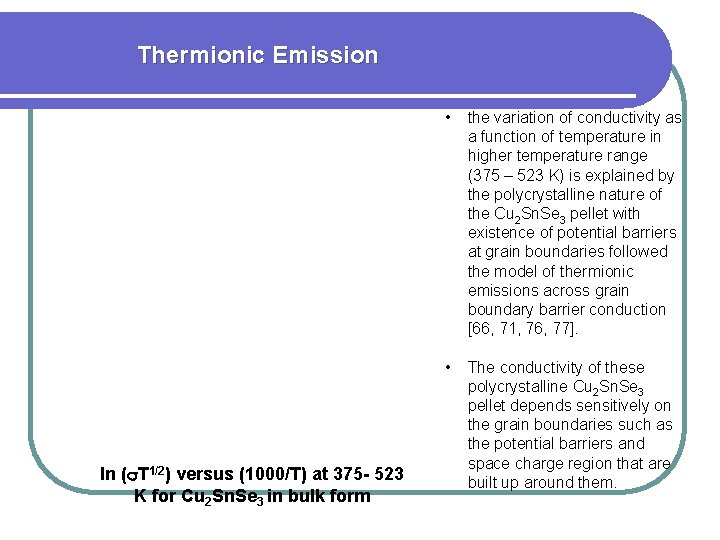
Thermionic Emission ln ( T 1/2) versus (1000/T) at 375 - 523 K for Cu 2 Sn. Se 3 in bulk form • the variation of conductivity as a function of temperature in higher temperature range (375 – 523 K) is explained by the polycrystalline nature of the Cu 2 Sn. Se 3 pellet with existence of potential barriers at grain boundaries followed the model of thermionic emissions across grain boundary barrier conduction [66, 71, 76, 77]. • The conductivity of these polycrystalline Cu 2 Sn. Se 3 pellet depends sensitively on the grain boundaries such as the potential barriers and space charge region that are built up around them.
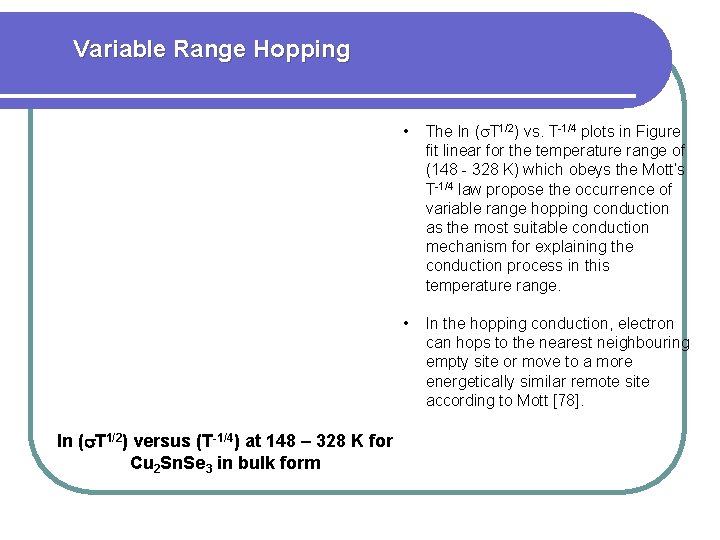
Variable Range Hopping ln ( T 1/2) versus (T-1/4) at 148 – 328 K for Cu 2 Sn. Se 3 in bulk form • The ln ( T 1/2) vs. T-1/4 plots in Figure fit linear for the temperature range of (148 - 328 K) which obeys the Mott’s T-1/4 law propose the occurrence of variable range hopping conduction as the most suitable conduction mechanism for explaining the conduction process in this temperature range. • In the hopping conduction, electron can hops to the nearest neighbouring empty site or move to a more energetically similar remote site according to Mott [78].
Thermal Properties
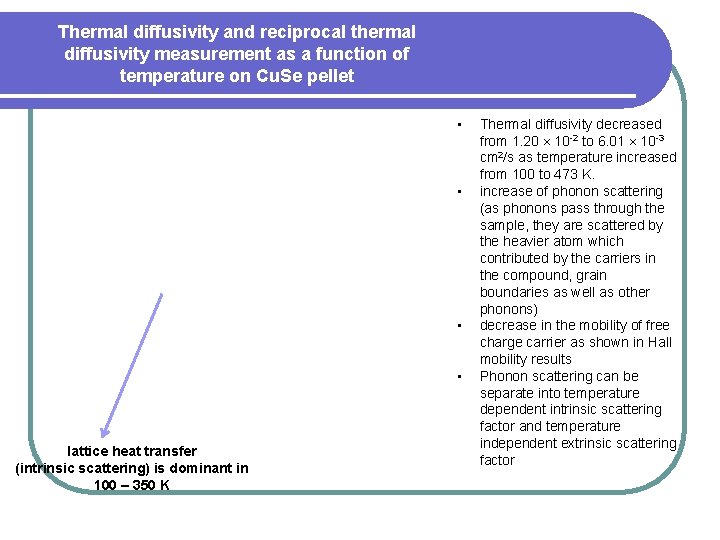
Thermal diffusivity and reciprocal thermal diffusivity measurement as a function of temperature on Cu. Se pellet • • lattice heat transfer (intrinsic scattering) is dominant in 100 – 350 K Thermal diffusivity decreased from 1. 20 10 -2 to 6. 01 10 -3 cm 2/s as temperature increased from 100 to 473 K. increase of phonon scattering (as phonons pass through the sample, they are scattered by the heavier atom which contributed by the carriers in the compound, grain boundaries as well as other phonons) decrease in the mobility of free charge carrier as shown in Hall mobility results Phonon scattering can be separate into temperature dependent intrinsic scattering factor and temperature independent extrinsic scattering factor
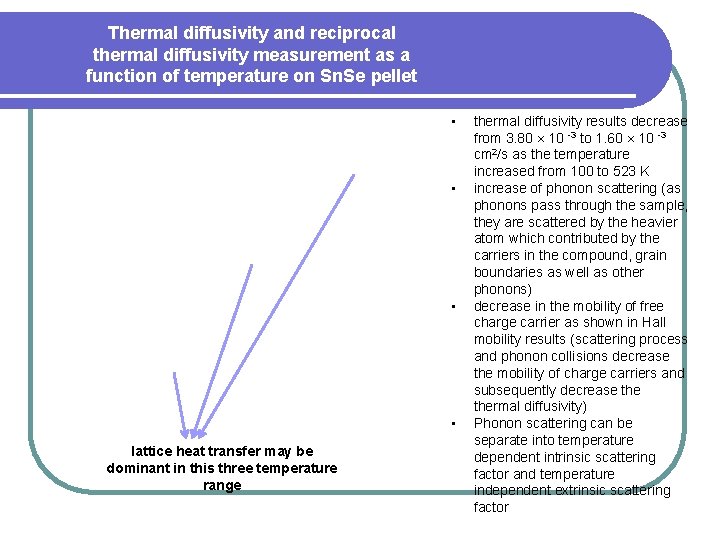
Thermal diffusivity and reciprocal thermal diffusivity measurement as a function of temperature on Sn. Se pellet • • lattice heat transfer may be dominant in this three temperature range thermal diffusivity results decrease from 3. 80 10 -3 to 1. 60 10 -3 cm 2/s as the temperature increased from 100 to 523 K increase of phonon scattering (as phonons pass through the sample, they are scattered by the heavier atom which contributed by the carriers in the compound, grain boundaries as well as other phonons) decrease in the mobility of free charge carrier as shown in Hall mobility results (scattering process and phonon collisions decrease the mobility of charge carriers and subsequently decrease thermal diffusivity) Phonon scattering can be separate into temperature dependent intrinsic scattering factor and temperature independent extrinsic scattering factor
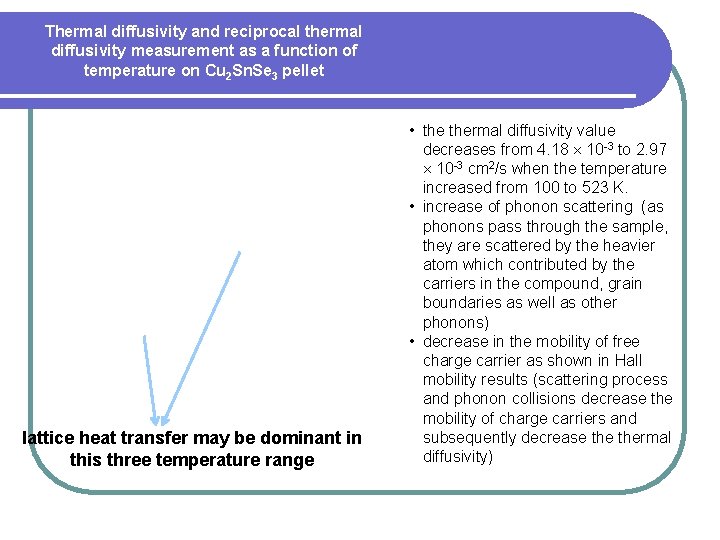
Thermal diffusivity and reciprocal thermal diffusivity measurement as a function of temperature on Cu 2 Sn. Se 3 pellet lattice heat transfer may be dominant in this three temperature range • thermal diffusivity value decreases from 4. 18 10 -3 to 2. 97 10 -3 cm 2/s when the temperature increased from 100 to 523 K. • increase of phonon scattering (as phonons pass through the sample, they are scattered by the heavier atom which contributed by the carriers in the compound, grain boundaries as well as other phonons) • decrease in the mobility of free charge carrier as shown in Hall mobility results (scattering process and phonon collisions decrease the mobility of charge carriers and subsequently decrease thermal diffusivity)
Effect of annealing process Cu. Se film
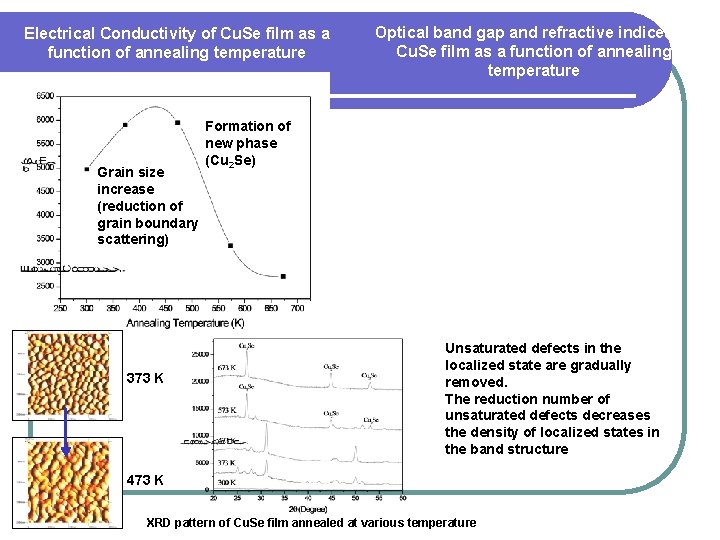
Electrical Conductivity of Cu. Se film as a function of annealing temperature Grain size increase (reduction of grain boundary scattering) 373 K Optical band gap and refractive indices of Cu. Se film as a function of annealing temperature Formation of new phase (Cu 2 Se) Unsaturated defects in the localized state are gradually removed. The reduction number of unsaturated defects decreases the density of localized states in the band structure 473 K XRD pattern of Cu. Se film annealed at various temperature
Effect of annealing process Sn. Se Thin Film
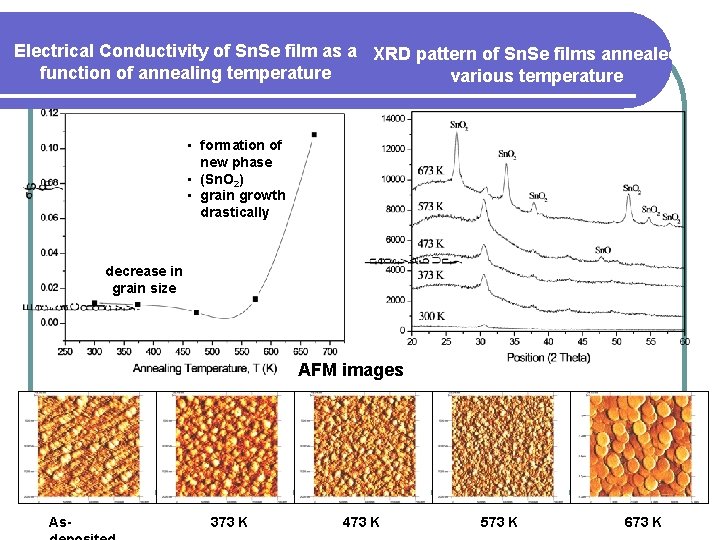
Electrical Conductivity of Sn. Se film as a XRD pattern of Sn. Se films annealed at function of annealing temperature various temperature • formation of new phase • (Sn. O 2) • grain growth drastically decrease in grain size AFM images As- 373 K 473 K 573 K 673 K
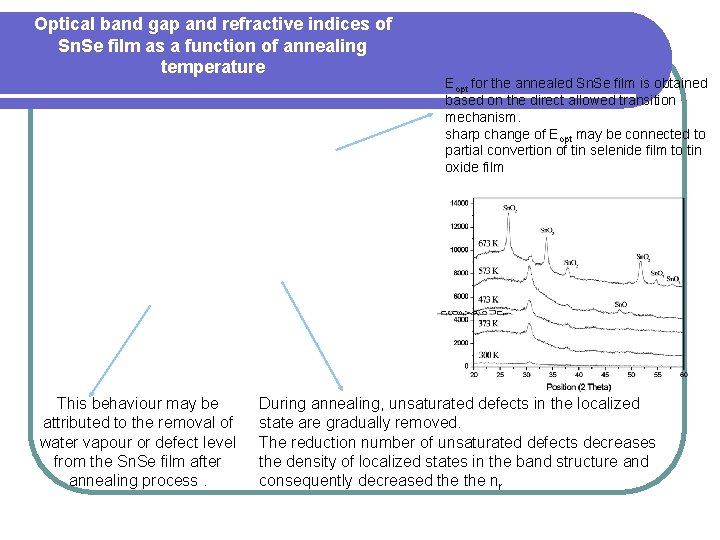
Optical band gap and refractive indices of Sn. Se film as a function of annealing temperature This behaviour may be attributed to the removal of water vapour or defect level from the Sn. Se film after annealing process. Eopt for the annealed Sn. Se film is obtained based on the direct allowed transition mechanism. sharp change of Eopt may be connected to partial convertion of tin selenide film to tin oxide film During annealing, unsaturated defects in the localized state are gradually removed. The reduction number of unsaturated defects decreases the density of localized states in the band structure and consequently decreased the nr
Effect of annealing process Cu 2 Sn. Se 3 Thin Film
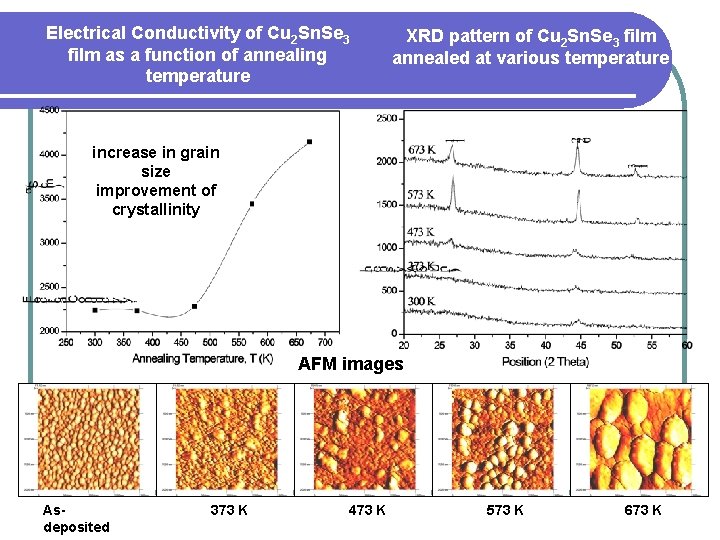
Electrical Conductivity of Cu 2 Sn. Se 3 film as a function of annealing temperature XRD pattern of Cu 2 Sn. Se 3 film annealed at various temperature increase in grain size improvement of crystallinity AFM images Asdeposited 373 K 473 K 573 K 673 K
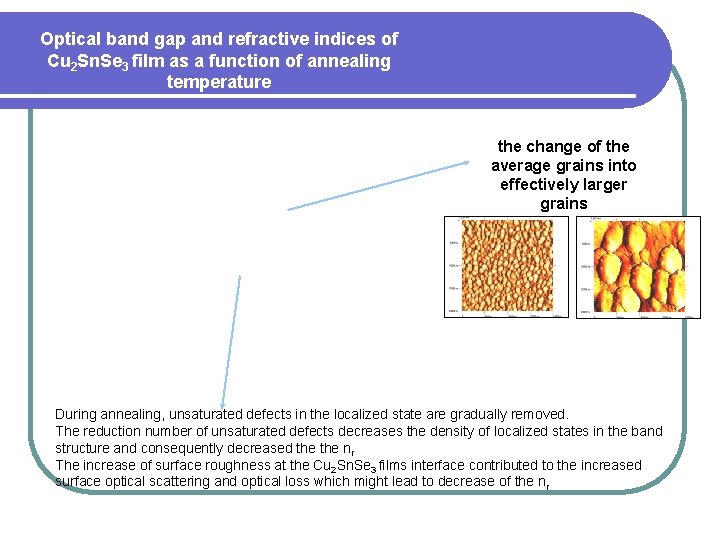
Optical band gap and refractive indices of Cu 2 Sn. Se 3 film as a function of annealing temperature the change of the average grains into effectively larger grains During annealing, unsaturated defects in the localized state are gradually removed. The reduction number of unsaturated defects decreases the density of localized states in the band structure and consequently decreased the nr The increase of surface roughness at the Cu 2 Sn. Se 3 films interface contributed to the increased surface optical scattering and optical loss which might lead to decrease of the nr
REFERENCES 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. Loferski, J. J. J. Appl. Phys. , 1956. 27(7): 777 -784. Kainthla, R. C. , Pandya, D. K. , Chopra, K. L. J. Electrochem. Soc. , 1982. 129(1): 99 -102. Lakshmikumar, S. T. , Rastogi, A. C. Sol. Energy Mater. Sol. Cells, 1994. 32(1): 7 -19. Grozdanov, I. Synth. Met. , 1994. 63(3): 213 -216. Li, B. , Xie, Y. , Huang, J. , Qian, Y. Ultrason. Sonochem. , 1999. 6(4): 217 -220. Bhuse, V. M. , Hankare, P. P. , Garadkar, K. M. , Khomane, A. S. Mater. Chem. Phys. , 2003. 80(1): 82 -88. Dhanam, M. , Manoj, P. K. , Prabhu, R. R. J. Cryst. Growth, 2005. 280(3 -4): 425 -435. Zhang, S. Y. , Fang, C. X. , Tian, Y. P. , Zhu, K. R. , Jin, B. K. , Shen, Y. H. , Yang, J. X. Cryst. Growth Des. , 2006. 6(12): 28092813. Ambade, S. B. , Mane, R. S. , Kale, S. S. , Sonawane, S. H. , Shaikh, A. V. , Han, S. -H. Appl. Surf. Sci. , 2006. 253(4): 21232126. Seoudi, R. , Shabaka, A. A. , Elokr, M. M. , Sobhi, A. Mater. Lett. , 2007. 61(16): 3451 -3455. Gosavi, S. R. , Deshpande, N. G. , Gudage, Y. G. , Sharma, R. J. Alloys Compd. , 2008. 448(1 -2): 344 -348. Hankare, P. P. , Khomane, A. S. , Chate, P. A. , Rathod, K. C. , Garadkar, K. M. J. Alloys Compd. , 2009. 469(1 -2): 478 -482. Hermann, A. M. , Fabick, L. J. Cryst. Growth, 1983. 61(3): 658 -664. Chu, T. L. , Chu, S. S. , Lin, S. C. , Yue, J. J. Electrochem. Soc. , 1984. 131(9): 2182 -2185. O'Brien, R. N. , Santhanam, K. S. V. J. Electrochem. Soc. , 1992. 139(2): 434 -437. Bhattacharya, R. N. , Fernandez, A. M. , Contreras, M. A. , Keane, J. , Tennant, A. L. , Ramanathan, K. , Tuttle, J. R. , Noufi, R. N. , Hermann, A. M. J. Electrochem. Soc. , 1996. 143(3): 854 -858. Lakshmi, M. , Bindu, K. , Bini, S. , Vijayakumar, K. P. , Kartha, C. S. , Abe, T. , Kashiwaba, Y. Thin Solid Films, 2001. 386(1): 127 -132. Pathan, H. M. , Lokhande, C. D. , Amalnerkar, D. P. , Seth, T. Appl. Surf. Sci. , 2003. 211(1 -4): 48 -56. Padam, G. K. Thin Solid Films, 1987. 150(1): L 89 -L 92. Haram, S. K. , Santhanam, K. S. V. Thin Solid Films, 1994. 238(1): 21 -26.
21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. Levy-Clement, C. , Neumann-Spallart, M. , Haram, S. K. , Santhanam, K. S. V. Thin Solid Films, 1997. 302(1 -2): 12 -16. Heyding, R. D. , Murray, R. M. Can. J. Chem. , 1976. 54(6): 841 -848 Estrada, C. A. , Nair, P. K. , Nair, M. T. S. , Zingaro, R. A. , Meyers, E. A. J. Electrochem. Soc. , 1994. 141(3): 802 -806. Garcia, V. M. , Nair, P. K. , Nair, M. T. S. J. Cryst. Growth, 1999. 203(1 -2): 113 -124. Taylor, C. A. , Underwood, F. A. Acta Crystallogr. , 1960. 13(4): 361 -362. Elliott, J. A. , Bicknell, J. A. , Collinge, R. G. Acta Crystallogr. , Sect. B: Struct. Crystallogr. Cryst. Chem. , 1969. 25(11): 2420 Al-Mamun, Islam, A. B. M. O. Int. J. Mod. Phys. B, 2004. 18(22): 3063 -3069. Agarwal, A. , Patel, P. D. , Lakshminarayana, D. J. Cryst. Growth, 1994. 142(3 -4): 344 -348. Chun, D. , Walser, R. M. , Bene, R. W. , Courtney, T. H. Appl. Phys. Lett. , 1974. 24(10): 479 -481 Baxter, C. R. , Mc. Lennan, W. D. J. Vac. Sci. Technol. , A, 1975. 12(1): 110 -113. Valiukonis, G. , Guseinova, D. A. , Keivaitb, G. , Sileika, A. Phys. Status Solidi B, 1986. 135(1): 299 -307. Ganesan, N. , Sivaramakrishnan, V. Semicond. Sci. Technol. , 1987. 2(8): 519. Wang, L. -S. , Niu, B. , Lee, Y. T. , Shirley, D. A. , Balasubramanian, K. J. Chem. Phys. , 1990. 92(2): 899 -908. John, K. J. , Pradeep, B. , Mathai, E. J. Mater. Sci. , 1994. 29(6): 1581 -1583. Xie, Y. , Su, H. , Li, B. , Qian, Y. Mater. Res. Bull. , 2000. 35(3): 459 -464. Lindgren, T. , Larsson, M. , Lindquist, S. -E. Sol. Energy Mater. Sol. Cells, 2002. 73(4): 377 -389. Zainal, Z. , Nagalingam, S. , Kassim, A. , Hussein, M. Z. , Yunus, W. M. M. Sol. Energy Mater. Sol. Cells, 2004. 81(2): 261 -268. Agarwal, A. , Chaki, S. H. , Lakshminarayana, D. Mater. Lett. , 2007. 61(30): 5188 -5190. Nabi, Z. , Kellou, A. , Mecabih, S. , Khalfi, A. , Benosman, N. Mater. Sci. Eng. , B, 2003. 98(2): 104 -115. Quan, D. T. Thin Solid Films, 1987. 149(2): 197 -203.
41. 42. 43. 44. 45. 46. 47. 48. 49. 50. 51. 52. 53. 54. 55. 56. 57. 58. 59. 60. Julien, C. , Eddrief, M. , Samaras, I. , Balkanski, M. Mater. Sci. Eng. , B, 1992. 15(1): 70 -72. Subramanian, B. , Mahalingam, T. , Sanjeeviraja, C. , Jayachandran, M. , Chockalingam, M. J. Thin Solid Films, 1999. 357(2): 119 -124. Spitzer, D. P. J. Phys. Chem. Solids, 1970. 31(1): 19 -40. Shay, J. L. , Wernick, J. H. Ternary chalcopyrite semiconductors: growth, electronic properties and applications; Pergamon: Oxford 1975. Kuhs, W. F. , Nitsche, R. , Scheunemann, K. Mater. Res. Bull. , 1979. 14(2): 241 -248. Samanta, L. K. , Ghosh, D. K. , Bhar, G. C. Phys. Status Solidi A, 1986. 93(1): K 51 -K 54. Samanta, L. K. Phys. Status Solidi A, 1987. 100(1): K 93 -K 97. Boldish, S. I. , White, W. B. Am. Mineral. , 1998. 83(7 -8): 865 -871 Chen, X. -a. , Wada, H. , Sato, A. , Mieno, M. J. Solid State Chem. , 1998. 139(1): 144 -151. Onoda, M. , Chen, X. -a. , Sato, A. , Wada, H. Mater. Res. Bull. , 2000. 35(9): 1563 -1570. Marcano, G. , Bracho, D. B. , Rincon, C. , Perez, G. S. , Nieves, L. J. Appl. Phys. , 2000. 88(2): 822 -828. Skoug, E. J. , Cain, J. D. , Morelli, D. T. J. Alloys Compd. , 2010. 506(1): 18 -21. Berger, L. I. , Prochukhan, V. D. Ternary diamond-like semiconductors; Consultants Bureau: New York. 1969. Feigelson, R. Jpn. J. Appl. Phys. , 1980. 19((Supplement 19 -3)): 371 -376. Marcano, G. , Rincon, C. , de Chalbaud, L. M. , Bracho, D. B. , Perez, G. S. J. Appl. Phys. , 2001. 90(4): 1847 -1853. Badrinarayanan, S. , Mandale, A. , Gunjikar, V. , Sinha, A. J. Mater. Sci. , 1986. 21(9): 3333 -3338. Kale, R. B. , Lokhande, C. D. Appl. Surf. Sci. , 2005. 252(4): 929 -938. Kale, R. B. , Lokhande, C. D. J. Phys. Chem. B, 2005. 109(43): 20288 -20294. Kazmerski, L. L. , Berry, W. B. , Allen, C. W. J. Appl. Phys. , 1972. 43(8): 3515 -3521. Hummel, R. E. Electronic Properties of Materials; 3 rd ed. ; Springer-Verlag: New York. 2001.
61. 62. 63. 64. 65. 66. 67. 68. 69. 70. 71. 72. 73. 74. 75. 76. 77. 78. Ghazanfar, U. , Siddiqi, S. A. , Abbas, G. Mater. Sci. Eng. , B, 2005. 118(1 -3): 132 -134. Petritz, R. L. Phys. Rev. , 1956. 104(6): 1508. Mahmoud, S. A. , Ashour, A. , Badawi, E. A. Appl. Surf. Sci. , 2006. 253(5): 2969 -2972. Prasad, M. , Pandit, A. K. , Ansari, T. H. , Singh, R. A. Mater. Chem. Phys. , 1991. 30(1): 13 -17. Berger, H. , Jäniche, G. , Grachovskaya, N. Phys. Status Solidi B, 1969. 33(1): 417 -424. Seto, J. Y. W. J. Appl. Phys. , 1975. 46(12): 5247 -5254. Thamilselvan, M. , Premnazeer, K. , Mangalaraj, D. , Narayandass, S. K. Physica B (Amsterdam, Neth. ), 2003. 337(1 -4): 404 -412. Mott, N. F. , Davis, E. A. Electronic process in non-crystalline materials; 2 nd ed. ; Clarendon Press: Oxford. 1979. Mott, N. F. Metal-insulator transitions; Taylor & Francis: London. 1974. Sharma, R. P. , Shukla, A. K. , Kapoor, A. K. , Srivastava, R. , Mathur, P. C. J. Appl. Phys. , 1985. 57(6): 2026 -2029. Baccarani, G. , Ricco, B. , Spadini, G. J. Appl. Phys. , 1978. 49(11): 5565 -5570. Bertran, E. , et al. J. Phys. D: Appl. Phys. , 1984. 17(8): 1679. Hafiz, M. M. , Othman, A. A. , Elnahass, M. M. , Al-Motasem, A. T. Physica B (Amsterdam, Neth. ), 2007. 390(1 -2): 286 -292. Shandalov, M. , Dashevsky, Z. , Golan, Y. Mater. Chem. Phys. , 2008. 112(1): 132 -135. Ireland, J. R. Ph. D. Thesis. Transport characteristics of novel degenerate semiconductor materials chalcogenide based thermoelectric systems and cadmium oxide based thin film transparent conducting oxide systems. Northwestern University, 2004. Pike, G. E. , Seager, C. H. J. Appl. Phys. , 1979. 50(5): 3414 -3422. Bernède, J. C. , Manai, N. , Morsli, M. , Pouzet, J. , Marie, A. M. Thin Solid Films, 1992. 214(2): 200 -206. Yildiz, A. , Alsaç, A. , Serin, T. , Serin, N. J. Mater. Sci. : Mater. Electron. , 2010. 1 -4.

ACKNOWLEDGEMENTS The authors would like to thank the Ministry of Education and Universiti Putra Malaysia for their financial support through (FRGS 5524428), (RUGS 9341400) and (Geran Putra 9433966)

Thank you
- Slides: 108