Understanding the Evidence Preventing Detecting Managing PreEclampsia Eclampsia

















- Slides: 46

Understanding the Evidence: Preventing, Detecting & Managing Pre-Eclampsia & Eclampsia

Objectives 1. Present evidence on pre-eclampsia and eclampsia (PE/E) interventions available for PE/E prevention, detection and management 2. Share emerging evidence and innovations on PE/E

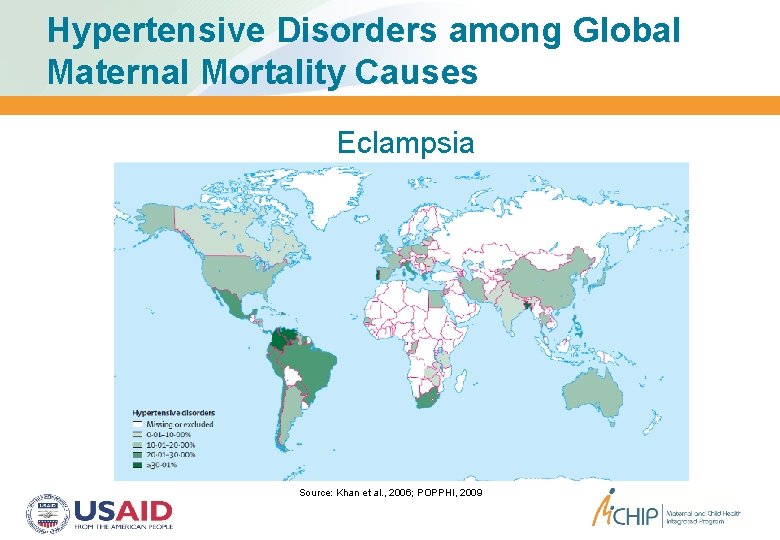
Hypertensive Disorders among Global Maternal Mortality Causes Eclampsia Source: Khan et al. , 2006; POPPHI, 2009

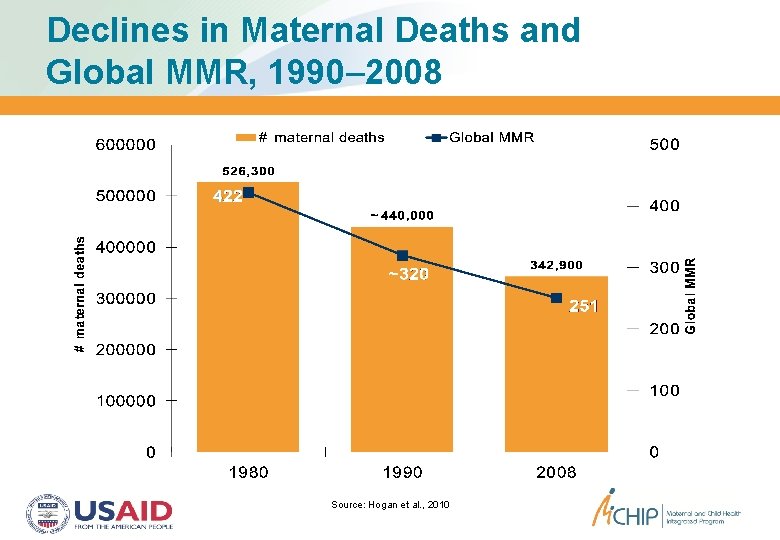
Declines in Maternal Deaths and Global MMR, 1990– 2008 Source: Hogan et al. , 2010

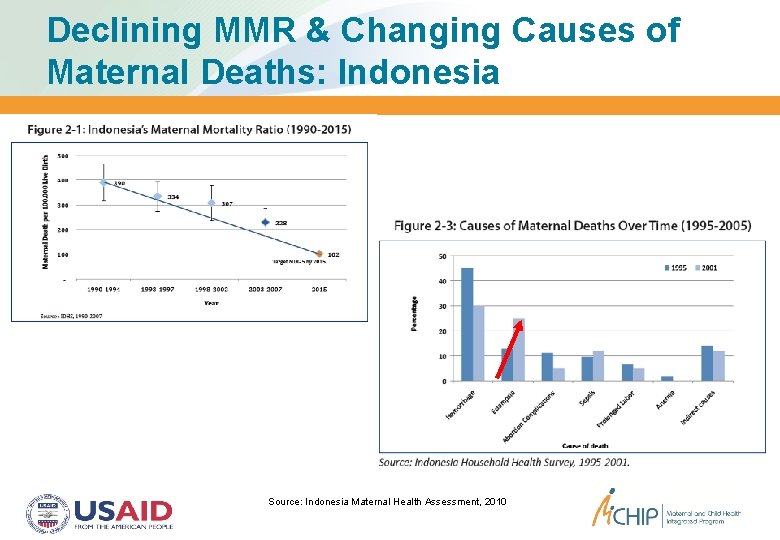
Declining MMR & Changing Causes of Maternal Deaths: Indonesia Source: Indonesia Maternal Health Assessment, 2010

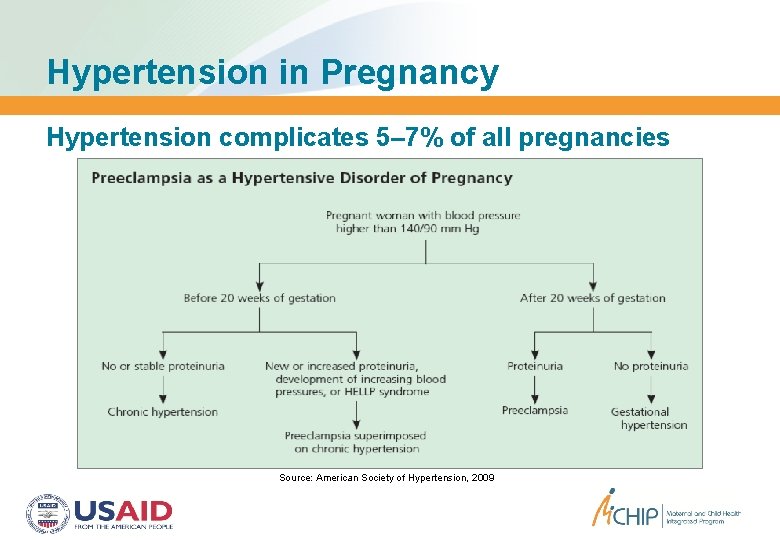
Hypertension in Pregnancy Hypertension complicates 5– 7% of all pregnancies Source: American Society of Hypertension, 2009

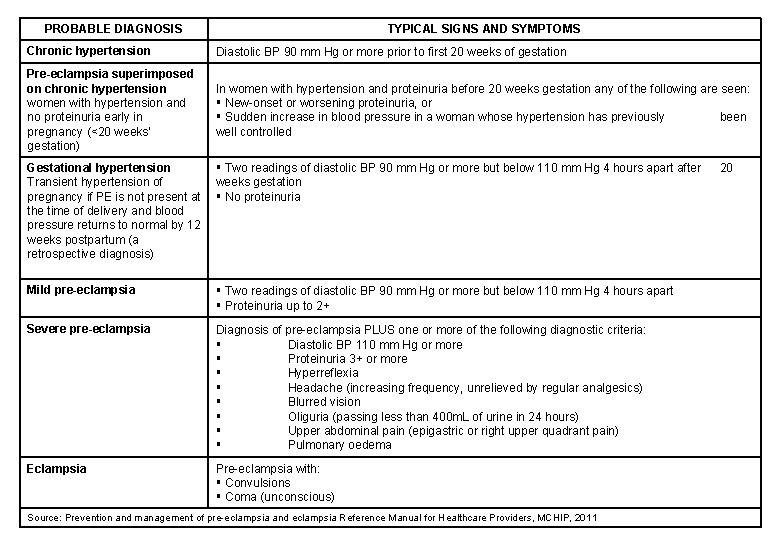
PROBABLE DIAGNOSIS TYPICAL SIGNS AND SYMPTOMS Chronic hypertension Diastolic BP 90 mm Hg or more prior to first 20 weeks of gestation Pre-eclampsia superimposed on chronic hypertension women with hypertension and no proteinuria early in pregnancy (<20 weeks’ gestation) In women with hypertension and proteinuria before 20 weeks gestation any of the following are seen: New-onset or worsening proteinuria, or Sudden increase in blood pressure in a woman whose hypertension has previously been well controlled Gestational hypertension Transient hypertension of pregnancy if PE is not present at the time of delivery and blood pressure returns to normal by 12 weeks postpartum (a retrospective diagnosis) Two readings of diastolic BP 90 mm Hg or more but below 110 mm Hg 4 hours apart after weeks gestation No proteinuria Mild pre-eclampsia Two readings of diastolic BP 90 mm Hg or more but below 110 mm Hg 4 hours apart Proteinuria up to 2+ Severe pre-eclampsia Diagnosis of pre-eclampsia PLUS one or more of the following diagnostic criteria: Diastolic BP 110 mm Hg or more Proteinuria 3+ or more Hyperreflexia Headache (increasing frequency, unrelieved by regular analgesics) Blurred vision Oliguria (passing less than 400 m. L of urine in 24 hours) Upper abdominal pain (epigastric or right upper quadrant pain) Pulmonary oedema Eclampsia Pre-eclampsia with: Convulsions Coma (unconscious) Source: Prevention and management of pre-eclampsia and eclampsia Reference Manual for Healthcare Providers, MCHIP, 2011 20

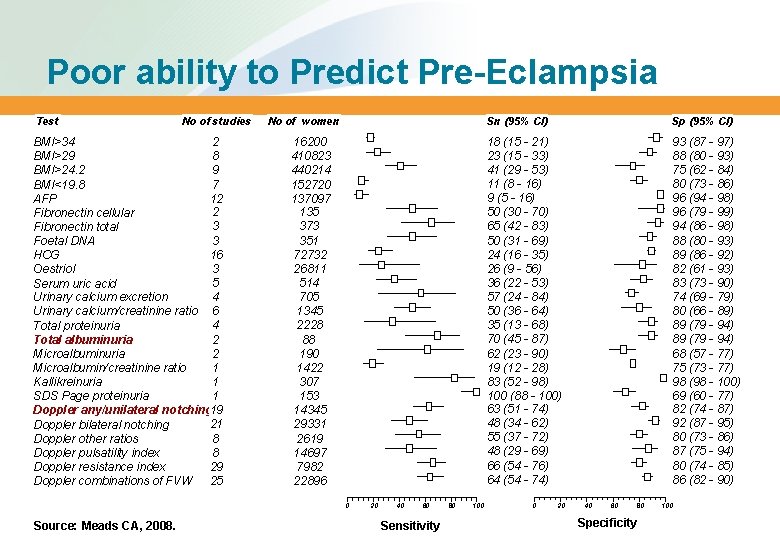
Poor ability to Predict Pre-Eclampsia Test No of studies BMI>34 2 BMI>29 8 9 BMI>24. 2 7 BMI<19. 8 12 AFP 2 Fibronectin cellular 3 Fibronectin total 3 Foetal DNA 16 HCG 3 Oestriol 5 Serum uric acid 4 Urinary calcium excretion Urinary calcium/creatinine ratio 6 4 Total proteinuria 2 Total albuminuria Microalbuminuria 2 Microalbumin/creatinine ratio 1 1 Kallikreinuria SDS Page proteinuria 1 Doppler any/unilateral notching 19 21 Doppler bilateral notching 8 Doppler other ratios 8 Doppler pulsatility index Doppler resistance index 29 Doppler combinations of FVW 25 No of women 16200 410823 440214 152720 137097 135 373 351 72732 26811 514 705 1345 2228 88 190 1422 307 153 14345 29331 2619 14697 7982 22896 0 Source: Meads CA, 2008. 20 40 60 Sensitivity 80 100 Sn (95% CI) Sp (95% CI) 18 (15 - 21) 23 (15 - 33) 41 (29 - 53) 11 (8 - 16) 9 (5 - 16) 50 (30 - 70) 65 (42 - 83) 50 (31 - 69) 24 (16 - 35) 26 (9 - 56) 36 (22 - 53) 57 (24 - 84) 50 (36 - 64) 35 (13 - 68) 70 (45 - 87) 62 (23 - 90) 19 (12 - 28) 83 (52 - 98) 100 (88 - 100) 63 (51 - 74) 48 (34 - 62) 55 (37 - 72) 48 (29 - 69) 66 (54 - 76) 64 (54 - 74) 93 (87 - 97) 88 (80 - 93) 75 (62 - 84) 80 (73 - 86) 96 (94 - 98) 96 (79 - 99) 94 (86 - 98) 88 (80 - 93) 89 (86 - 92) 82 (61 - 93) 83 (73 - 90) 74 (69 - 79) 80 (66 - 89) 89 (79 - 94) 68 (57 - 77) 75 (73 - 77) 98 (98 - 100) 69 (60 - 77) 82 (74 - 87) 92 (87 - 95) 80 (73 - 86) 87 (75 - 94) 80 (74 - 85) 86 (82 - 90) 0 20 40 60 80 Specificity 100

Who is at Increased Risk for PE? A personal or family history of PE/E Pre-existing medical condition including obesity, chronic hypertension, or diabetes Age: ≤ 19; >35 years Primigravida IUGR, abruption placenta or fetal death in previous pregnancy First pregnancy with a new partner All pregnant women potentially at risk. All need prevention and early detection of PE.

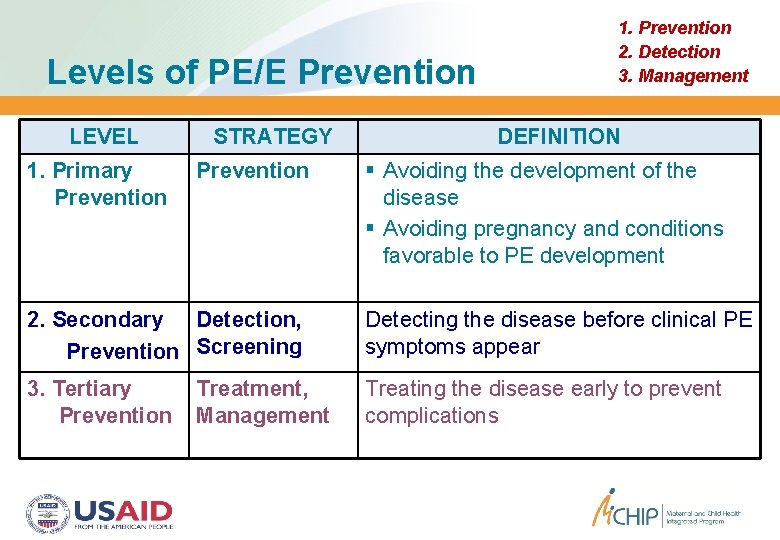
Levels of PE/E Prevention LEVEL 1. Primary Prevention STRATEGY Prevention 1. Prevention 2. Detection 3. Management DEFINITION Avoiding the development of the disease Avoiding pregnancy and conditions favorable to PE development 2. Secondary Detection, Prevention Screening Detecting the disease before clinical PE symptoms appear 3. Tertiary Prevention Treating the disease early to prevent complications Treatment, Management

PE/E Prevention 1. Prevention 2. Detection 3. Management Prevention and prediction are difficult because: The cause is not well understood The associated factors are difficult to influence Focus on symptomatic clinical management to prevent maternal morbidity (e. g. , eclampsia) and mortality Source: Steegers EA et al. , Lancet. 2010

Taking Evidence to Scale Photo credit: Daniel Antonaccio Seeking simple, inexpensive and effective solutions that reach all pregnant women 1. Prevention 2. Detection 3. Management

1. Prevention Preventing Pre-Eclampsia Almost 100 interventions tested in randomized trials x x x

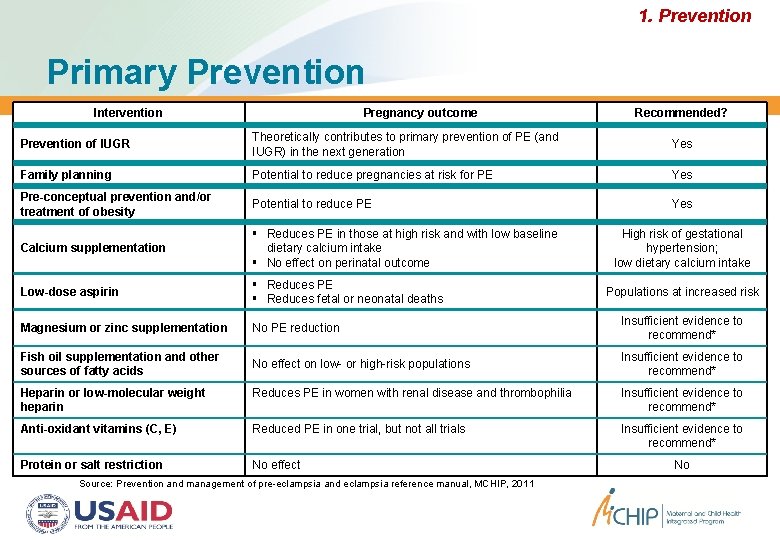
1. Prevention Primary Prevention Intervention Pregnancy outcome Recommended? Prevention of IUGR Theoretically contributes to primary prevention of PE (and IUGR) in the next generation Yes Family planning Potential to reduce pregnancies at risk for PE Yes Pre-conceptual prevention and/or treatment of obesity Potential to reduce PE Yes Calcium supplementation Reduces PE in those at high risk and with low baseline dietary calcium intake No effect on perinatal outcome Low-dose aspirin Reduces PE Reduces fetal or neonatal deaths Magnesium or zinc supplementation No PE reduction Insufficient evidence to recommend* Fish oil supplementation and other sources of fatty acids No effect on low- or high-risk populations Insufficient evidence to recommend* High risk of gestational hypertension; low dietary calcium intake Populations at increased risk Heparin or low-molecular weight heparin Reduces PE in women with renal disease and thrombophilia Insufficient evidence to recommend* Anti-oxidant vitamins (C, E) Reduced PE in one trial, but not all trials Insufficient evidence to recommend* Protein or salt restriction No effect Source: Prevention and management of pre-eclampsia and eclampsia reference manual, MCHIP, 2011 No

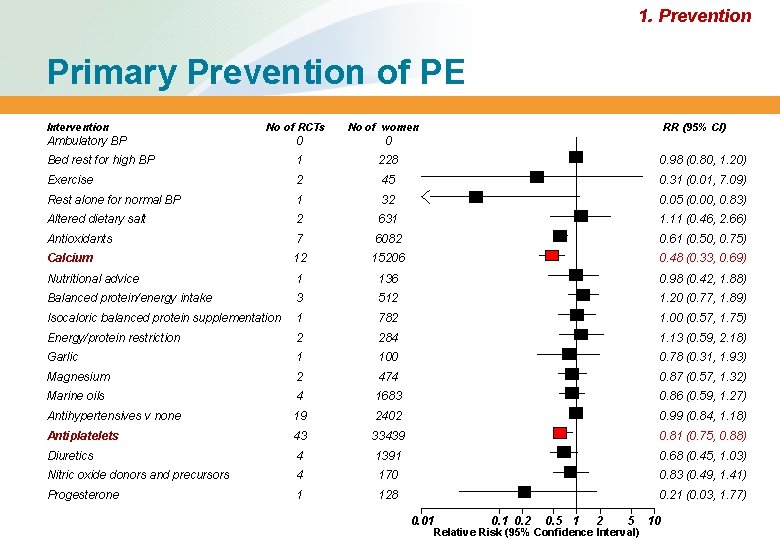
1. Prevention Primary Prevention of PE Intervention No of RCTs No of women RR (95% CI) Ambulatory BP 0 0 Bed rest for high BP 1 228 0. 98 (0. 80, 1. 20) Exercise 2 45 0. 31 (0. 01, 7. 09) Rest alone for normal BP 1 32 0. 05 (0. 00, 0. 83) Altered dietary salt 2 631 1. 11 (0. 46, 2. 66) Antioxidants 7 6082 0. 61 (0. 50, 0. 75) Calcium 12 15206 0. 48 (0. 33, 0. 69) Nutritional advice 1 136 0. 98 (0. 42, 1. 88) Balanced protein/energy intake 3 512 1. 20 (0. 77, 1. 89) Isocaloric balanced protein supplementation 1 782 1. 00 (0. 57, 1. 75) Energy/protein restriction 2 284 1. 13 (0. 59, 2. 18) Garlic 1 100 0. 78 (0. 31, 1. 93) Magnesium 2 474 0. 87 (0. 57, 1. 32) Marine oils 4 1683 0. 86 (0. 59, 1. 27) Antihypertensives v none 19 2402 0. 99 (0. 84, 1. 18) Antiplatelets 43 33439 0. 81 (0. 75, 0. 88) Diuretics 4 1391 0. 68 (0. 45, 1. 03) Nitric oxide donors and precursors 4 170 0. 83 (0. 49, 1. 41) Progesterone 1 128 0. 21 (0. 03, 1. 77) 0. 01 0. 2 0. 5 1 2 5 Relative Risk (95% Confidence Interval) 10

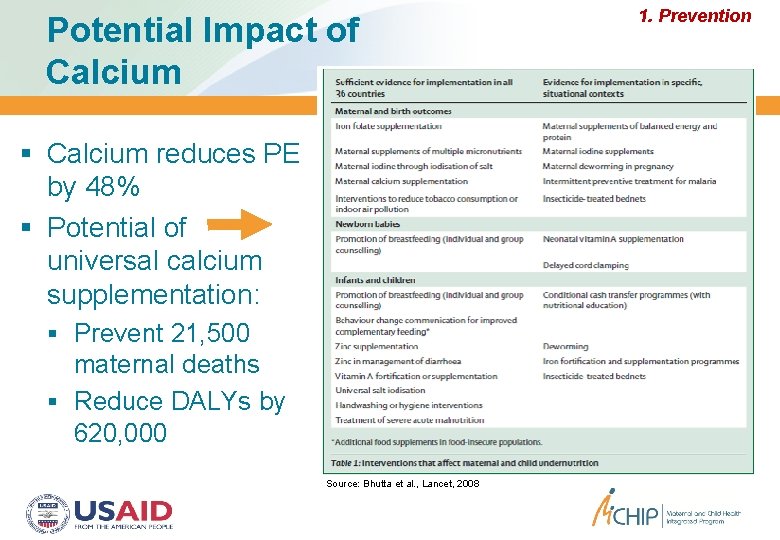
Potential Impact of Calcium reduces PE by 48% Potential of universal calcium supplementation: Prevent 21, 500 maternal deaths Reduce DALYs by 620, 000 Source: Bhutta et al. , Lancet, 2008 1. Prevention

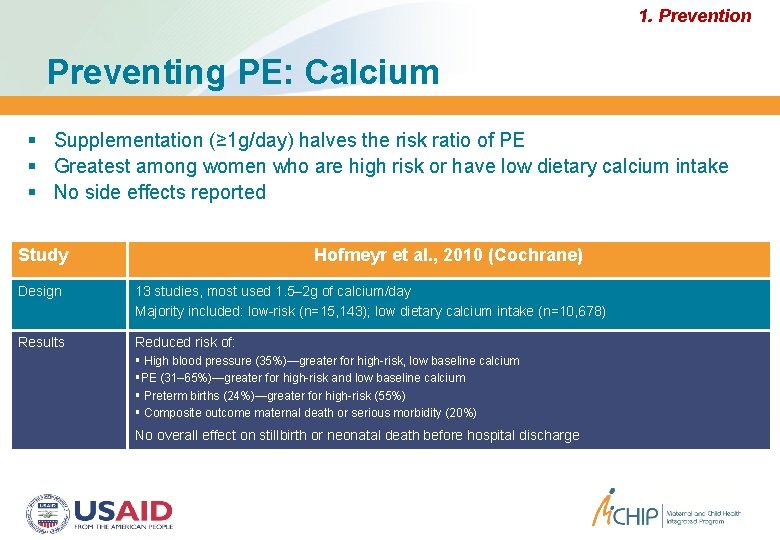
1. Prevention Preventing PE: Calcium Supplementation (≥ 1 g/day) halves the risk ratio of PE Greatest among women who are high risk or have low dietary calcium intake No side effects reported Study Hofmeyr et al. , 2010 (Cochrane) Design 13 studies, most used 1. 5– 2 g of calcium/day Majority included: low-risk (n=15, 143); low dietary calcium intake (n=10, 678) Results Reduced risk of: High blood pressure (35%)—greater for high-risk, low baseline calcium PE (31– 65%)—greater for high-risk and low baseline calcium Preterm births (24%)—greater for high-risk (55%) Composite outcome maternal death or serious morbidity (20%) No overall effect on stillbirth or neonatal death before hospital discharge

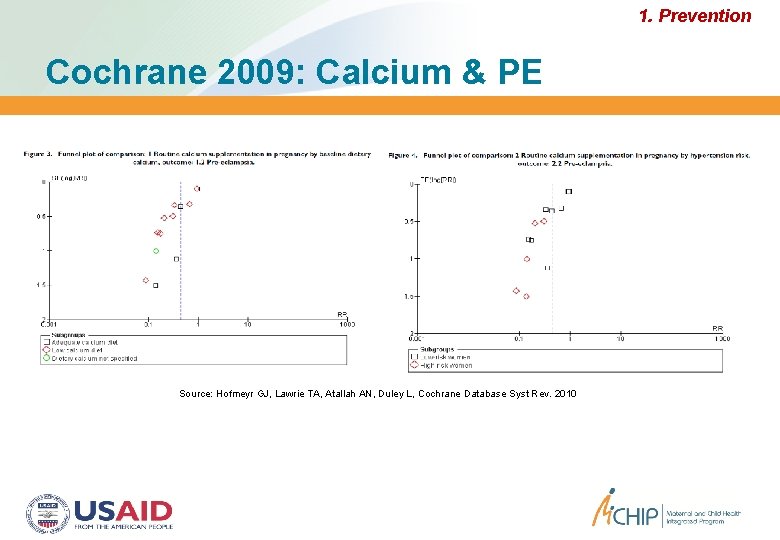
1. Prevention Cochrane 2009: Calcium & PE Source: Hofmeyr GJ, Lawrie TA, Atallah AN, Duley L, Cochrane Database Syst Rev. 2010

Preventing PE: Calcium, WHO Reproductive Health Library, 2010 1. Prevention Useful in low-resource settings Women with low habitual calcium intake appeared to benefit more No adverse effects, relatively safe Supports Cochrane findings calcium supplementation (>1 g/day) during pregnancy reduced risk, but interpret results with caution: greatest for women High BP Half as likely to get PE at high risk for PE; low baseline dietary calcium No evidence of significant difference for: Maternal outcomes (proteinuria, severe PE, eclampsia, maternal death) Perinatal/neonatal (preterm birth, LBW, SGA, stillbirth or death before discharge from hospital Source: RHL Commentary by Palacios C and Pena-Rosas JP, 2010

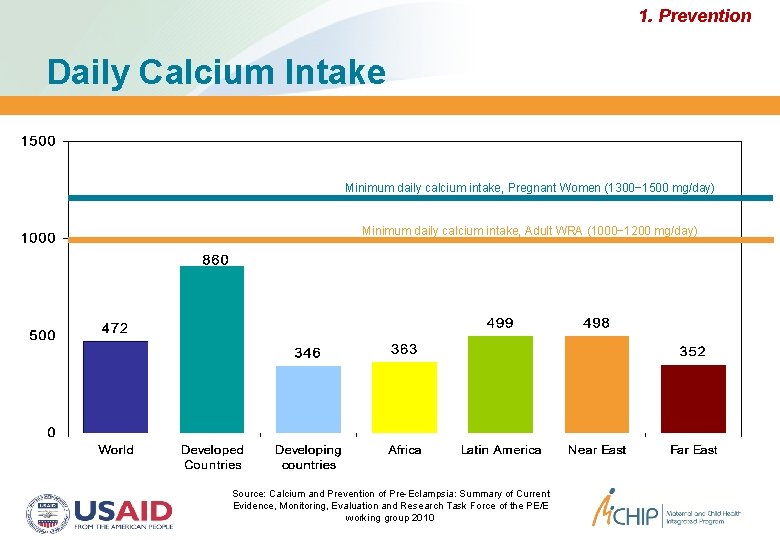
1. Prevention Daily Calcium Intake Minimum daily calcium intake, Pregnant Women (1300− 1500 mg/day) Minimum daily calcium intake, Adult WRA (1000− 1200 mg/day) Source: Calcium and Prevention of Pre-Eclampsia: Summary of Current Evidence, Monitoring, Evaluation and Research Task Force of the PE/E working group 2010

1. Prevention Photo credit: Paul Geor, www. sxc. hu Calcium & Iron Evidence (2005) Added calcium reduced the initial uptake of heme iron by 20% Reduced total iron absorbed by 25% Nonheme iron absorption not significantly affected “the long-term use of dietary calcium supplements… may further increase the risk of iron deficiency in women who are having difficulty in meeting their iron requirements. ” Implications (2010) Consider bioavailability of calcium from supplements: Solubility, size of the dose Interacts with iron, zinc, magnesium and phosphorus Inhibits iron absorption in a dosedependent and dose-saturable fashion separate time during the day from daily iron+folic acid supplementation Sources: Roughead, Z; Zito CA, Hunt JR 2005; RHL Commentary by Palacios C and Pena-Rosas JP, 2010

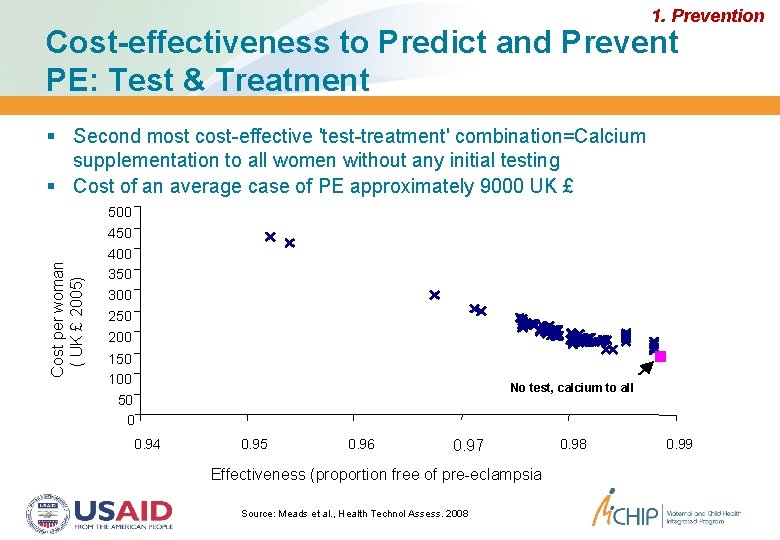
1. Prevention Cost-effectiveness to Predict and Prevent PE: Test & Treatment Second most cost-effective 'test-treatment' combination=Calcium supplementation to all women without any initial testing Cost of an average case of PE approximately 9000 UK £ Cost per woman ( UK £ 2005) 500 450 400 350 300 250 200 150 100 50 0 0. 94 No test, calcium to all 0. 95 0. 96 0. 97 Effectiveness (proportion free of pre-eclampsia Source: Meads et al. , Health Technol Assess. 2008 0. 99

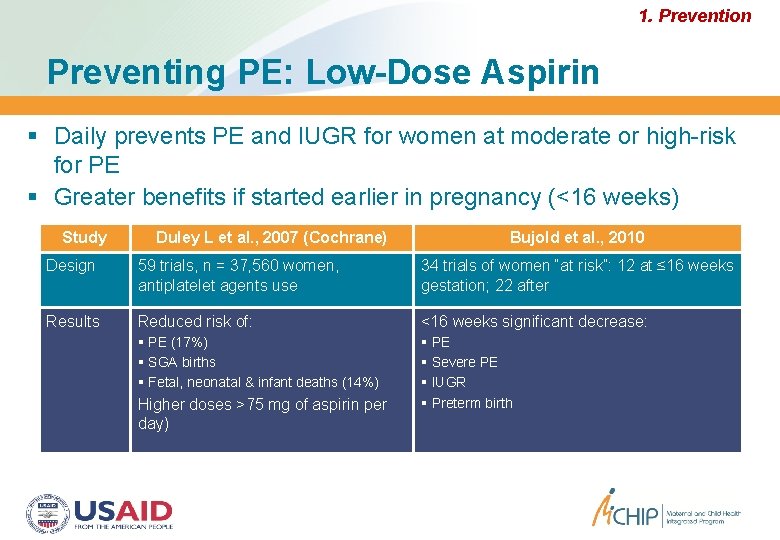
1. Prevention Preventing PE: Low-Dose Aspirin Daily prevents PE and IUGR for women at moderate or high-risk for PE Greater benefits if started earlier in pregnancy (<16 weeks) Study Duley L et al. , 2007 (Cochrane) Bujold et al. , 2010 Design 59 trials, n = 37, 560 women, antiplatelet agents use 34 trials of women “at risk”: 12 at ≤ 16 weeks gestation; 22 after Results Reduced risk of: <16 weeks significant decrease: PE (17%) SGA births Fetal, neonatal & infant deaths (14%) PE Severe PE IUGR Preterm birth Higher doses >75 mg of aspirin per day)

1. Prevention Preventing PE: Vitamins C & E Oxidative stress = Underlying mechanism for PE/E? Vitamins C & E for pregnant women at high-risk for PE Communities at risk of poor nutritional status in developing countries 14– 22 weeks gestation, daily supplements of vitamin C (1000 mg) & E (400 iu), n = 1365 Did not prevent PE, eclampsia, gestational hypertension, LBW, SGA or perinatal deaths Source: Villar J et al. , BJOG 2009

1. Prevention Preventing PE: Vitamin D Deficiency in pregnancy: Associated with adverse maternal and fetal outcomes Worldwide epidemic (18– 84%) Linkage to calcium absorption which increases during pregnancy, peaking in the third trimester Recent studies found: Vitamin D deficiency <22 weeks is an independent predictor of PE Vitamin D plus calcium supplementation started at 20– 24 weeks significantly reduced BP but not PE Daily vitamin D intake (10– 15 g/day) in Norway reduced the adjusted risk for PE by 29% when adjusting for maternal BMI Source: Haugen M et al. , Epidemiology 2009; Mulligan et al. , Am J Obstet Gynecol. 2010

2. Detection Detecting Pre-Eclampsia No clinically-useful screening test to predict PE in either highrisk or low-risk groups (2004) Doppler ultrasonography 24 -hour ambulatory blood pressure Placental and fetal peptides Renal dysfunction-related tests Endothelial and oxidant stress dysfunction-related tests Ideal predictive test: Simple, innocuous, rapid, inexpensive, reproducible, and noninvasive Easy to perform early in pregnancy Source: Conde-Agudelo A, Villar J, Lindheimer M. Obstet Gynecol. 2004

2. Detection Detecting Pre-Eclampsia: Measuring BP Hypertension 10% of pregnancies, >20 weeks Diastolic BP 90 mm Hg 4 ANC visits/pregnancy BP history and measurement at each visit Accuracy Significant training needed to do BP well Robust and maintained equipment Photo credit: Sheena Currie Most common: high BP before proteinuria WHO ANC Guidelines

2. Detection Detecting Pre-Eclampsia: Proteinuria Hypertension with proteinuria associated with poorer maternal and perinatal outcomes Proteinuria among women with: Higher antenatal BP Deliver earlier More often require operative delivery Urine dipstick test: Rapid, simple Boiling: Not feasible in high volume sites Esbach: time-consuming, inpatient Source: Thornton CE et al. , Clin Exp Pharmacol Physiol 2010; Thangaratinam S et al. , BMC Med. 2009 Photo credit: Daniel Antonaccio Magnitude of proteinuria is a poor predictor of the major maternal and fetal complications Available tests:

2. Detection Dipstick Urine Testing for Protein Limited in reliability, sensitivity, specificity, and predictive value ANC screening in South Africa— missed a significant number of patients with proteinuria Widely used Only test available in low-income and middle-income countries Source: Steegers EA et al. , Lancet. 2010; Gangaram R, Ojwang PJ, Moodley J, Maharaj D. Hypertens Pregnancy. 2005 Photo credit: Daniel Antonaccio False negative rate of 48. 6% during

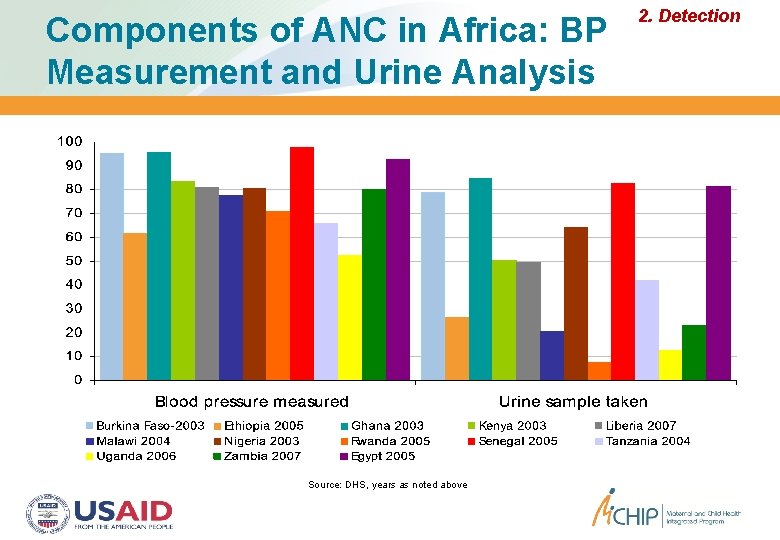
Components of ANC in Africa: BP Measurement and Urine Analysis Source: DHS, years as noted above 2. Detection

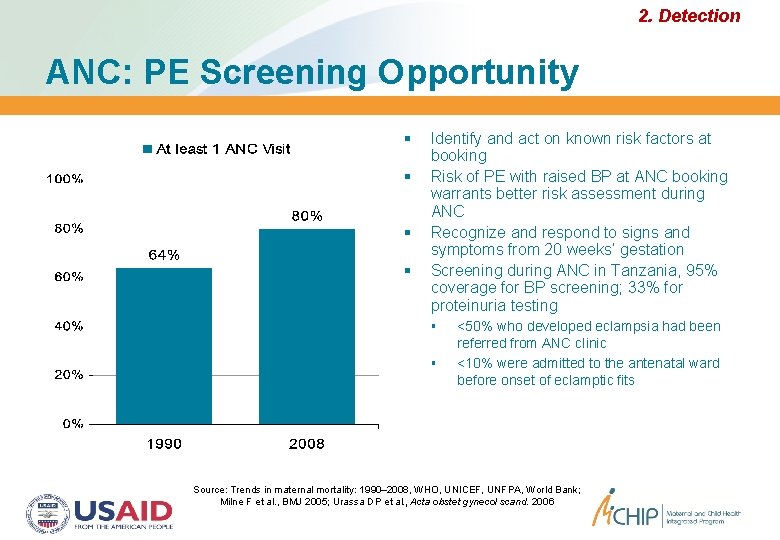
2. Detection ANC: PE Screening Opportunity Identify and act on known risk factors at booking Risk of PE with raised BP at ANC booking warrants better risk assessment during ANC Recognize and respond to signs and symptoms from 20 weeks’ gestation Screening during ANC in Tanzania, 95% coverage for BP screening; 33% for proteinuria testing <50% who developed eclampsia had been referred from ANC clinic <10% were admitted to the antenatal ward before onset of eclamptic fits Source: Trends in maternal mortality: 1990– 2008, WHO, UNICEF, UNFPA, World Bank; Milne F et al. , BMJ 2005; Urassa DP et al. , Acta obstet gynecol scand. 2006

3. Management Managing PE/E Anticonvulsants—magnesium sulfate Antihypertensives Timed delivery Clinical monitoring and vigilance x Diazepam x Lytic Cocktail x Rectal Avertin

3. Management Magnesium Sulfate: Treat Eclampsia Collaborative Eclampsia Trial, 1991– 1992, 27 centers in 9 countries, n =1680 Compared 3 most popular treatments Magnesium sulfate Diazepam Phenytoin Magnesium sulfate had a 52% and 67% lower recurrence of convulsions than diazepam and phenytoin respectively Source: The Eclampsia Trial Collaborative Group Lancet 1995

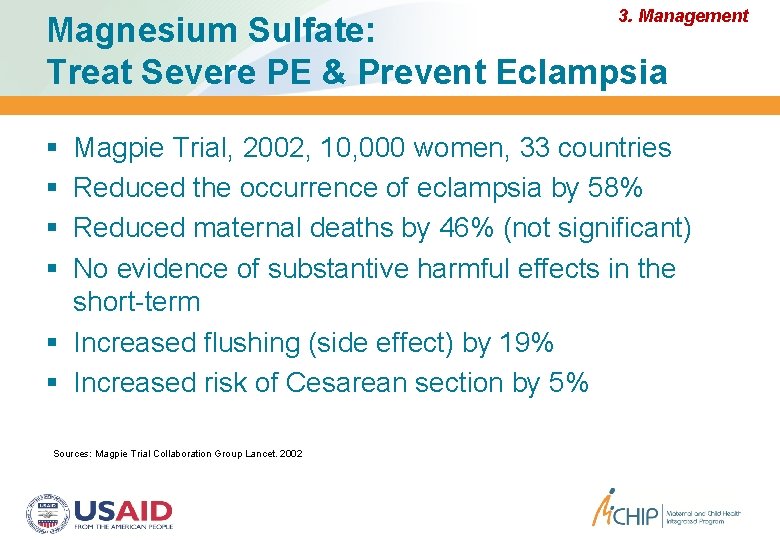
3. Management Magnesium Sulfate: Treat Severe PE & Prevent Eclampsia Magpie Trial, 2002, 10, 000 women, 33 countries Reduced the occurrence of eclampsia by 58% Reduced maternal deaths by 46% (not significant) No evidence of substantive harmful effects in the short-term Increased flushing (side effect) by 19% Increased risk of Cesarean section by 5% Sources: Magpie Trial Collaboration Group Lancet. 2002

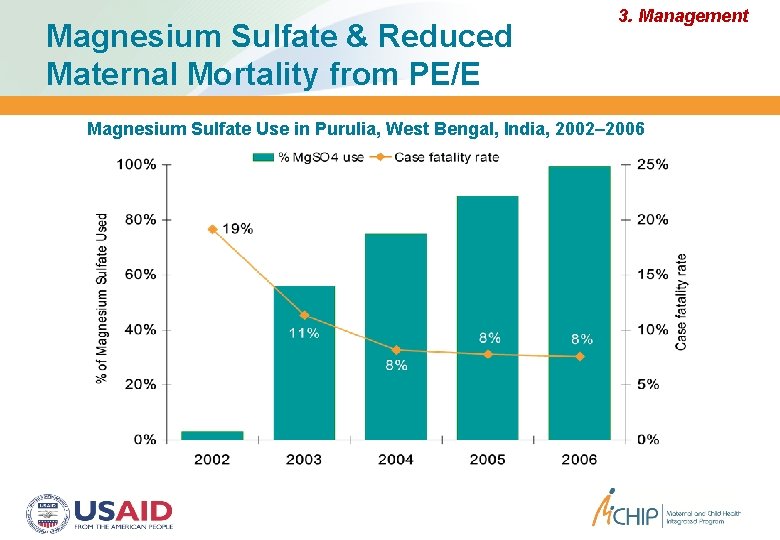
Magnesium Sulfate & Reduced Maternal Mortality from PE/E 3. Management Magnesium Sulfate Use in Purulia, West Bengal, India, 2002– 2006

3. Management Preventing Recurrent Convulsions Compared magnesium sulphate and diazepam 7 trials, 1441 women Reduced: Recurrence of convulsions Maternal mortality Apgar scores <7 at 1 and 5 minutes Other reviews confirm magnesium sulfate better than phenytoin or lytic cocktail Magnesium sulfate saves more mothers’ lives than diazepam when given for eclamptic fits. For every 7 women treated with magnesium sulfate (vs diazepam), 1 case of recurrent convulsions prevented Source: Duley L, Henderson-Smart D. Cochrane Database Syst Rev. 2003

3. Management Magnesium Sulfate and the Neonate Better outcomes for babies of mothers who received magnesium sulfate for eclampsia (than diazepam or phenytoin) Greater vigor of the babies (5 minutes after birth) Lower chances of a long hospital stay in an intensive care unit Fewer neonatal admissions to a special care unit Shorter duration of stay (in days) in the neonatal care unit Fewer neonatal deaths Source: Duley et al. , 2003 a

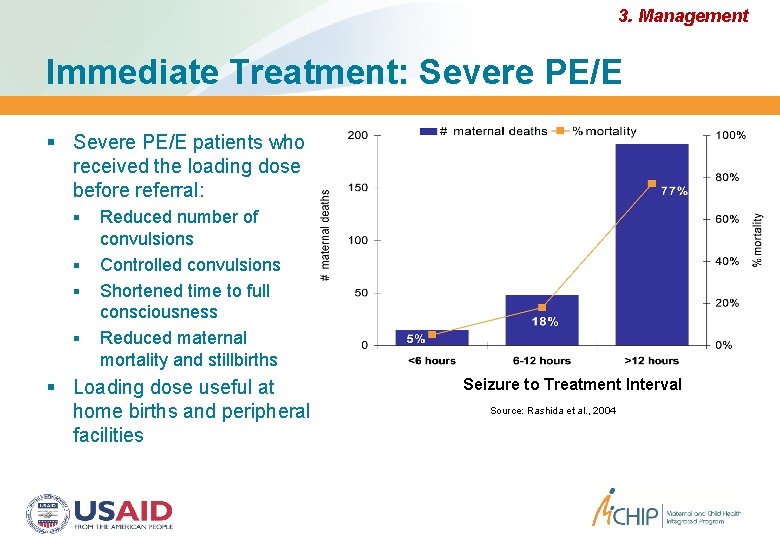
3. Management Immediate Treatment: Severe PE/E patients who received the loading dose before referral: Reduced number of convulsions Controlled convulsions Shortened time to full consciousness Reduced maternal mortality and stillbirths Loading dose useful at home births and peripheral facilities Seizure to Treatment Interval Source: Rashida et al. , 2004

3. Management Immediate Treatment: Eclampsia The sooner treatment starts, the better the survival rates Treatment is relatively simple if instituted immediately Magnesium sulfate Antihypertensive Delivery Delayed treatment especially beyond 2 hours requires intensive care for shock: DIC, renal shutdown, respiratory failure, electrolyte disturbance, sepsis, pneumonia, multi organ failure Even in best centers, mortality is high Ensure magnesium sulfate loading dose IM at the most peripheral healthcare facilities— including for homebirth. It maybe all that you need for safe transfer.

3. Management Standard Magnesium Sulfate Regimens 1. IV: 4 g loading dose over 10 to 15 minutes followed by infusion of 1 g/hour over 24 hours 2. IM: 4 g IV and 10 g IM as loading dose followed by 5 g IM every four hours for 24 hours Source: Duley L, Matar HE, Almerie MQ, Hall DR. Cochrane Database Syst Rev. 2010

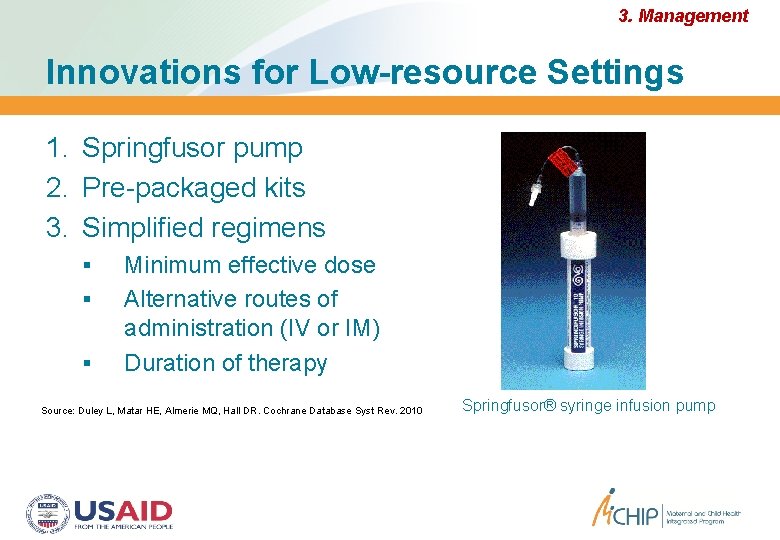
3. Management Innovations for Low-resource Settings 1. Springfusor pump 2. Pre-packaged kits 3. Simplified regimens Minimum effective dose Alternative routes of administration (IV or IM) Duration of therapy Source: Duley L, Matar HE, Almerie MQ, Hall DR. Cochrane Database Syst Rev. 2010 Springfusor® syringe infusion pump

Single Dose for Treatment of Eclampsia: Bangladesh 3. Management A randomized trial, 401 patients Efficacy of loading dose vs. standard regime Recurrent convulsion rate 4% vs 3. 5% Case fatality rate 4. 5% vs 5% Better outcomes for women receiving a loading dose at the community level before referral to a hospital (compared to those who received their loading dose in the hospital) Single loading dose sufficient Possible to treat—even at home

3. Management Magnesium Sulfate: Challenges 1. No policies to promote use: Lack of guidelines mandating the use Not on national essential drugs list 2. No information: if in national guidelines, not widely disseminated or mandatory 3. Available only at highest-level facilities because of perceived need for close monitoring 4. Health workers are commonly not trained or authorized to administer magnesium sulfate; lack confidence and knowledge 5. Rare and inexpensive=no incentive for drug companies 6. Inconvenient packs of 500– 1000 m. L; only 250 m. L needed Source: Reducing eclampsia-related deaths—a call to action, the Lancet, 2008

3. Management PE/E Management: Antihypertensives Reduce maternal risk without harming the fetus Help extend the pregnancy to improve fetal maturity and outcomes. Indicated when the diastolic pressure is >110 mm Hg Aim to bring it to 90– 100 mm Hg to prevent cerebral hemorrhage No clear choice of drugs Labetolol, hydralazine, and nifedipine currently widely recommended Once severe PE or eclampsia is diagnosed, at least the first dose of anti-hypertensive medications prior to transfer

3. Management Managing PE/E: Timed Delivery Induction of labor Associated with improved maternal outcome: • Mild gestational hypertension • >37 weeks gestation WHO Guidelines • Severe PE: Deliver <24 hours • Eclamptic convulsions/fits: Deliver <12 hours Expectant management with early onset severe PE Gained a mean of 11 days gestation with improved perinatal and neonatal survival rates Should not preclude timely delivery—the only definitive cure Sources: Steegers EA et al. , Lancet. 2010; Sibai BM, Barton JR Am J Obstet Gynecol. 2007

On the Horizon: 2003… 2011? 1. Prevention 2. Detection 3. Management “The technologies identified 5 years ago continue to be the key issues” Nutritional supplements to prevent PE/E Antiplatelets to prevent PE/E Methods for early detection of PE/E or elevated risk for PE/E Scaling up use of magnesium sulfate for both prevention and treatment of eclampsia Source: Tsu and Coffey, BJOG, 2009