Understanding Research Monitor Access to Cerner Power Chart








![Missing Consent Date Missing active start date for subject: [MRN number] • A consent Missing Consent Date Missing active start date for subject: [MRN number] • A consent](https://slidetodoc.com/presentation_image_h2/6cdaceaf031a37fdf4bc4b74e68bc704/image-17.jpg)



- Slides: 24

“Understanding Research Monitor Access to Cerner Power. Chart” IU Health Research Access Team 1/12/2022 1

Agenda • Overview • OCR Website- FAQ • REDCap Access Database • Research Viewpoint • Troubleshooting • Questions

Research Monitor Access Overview • New Policy • Patient lists replaced by Power. Chart Research Viewpoint enrolled subject list. • Access requested by the research staff via a REDCap survey and provisioned for the life of the trial. • Monitors will need to take annual mandatory training • On. Core and Power. Trials must be kept in sync.

RESEARCH MONITOR ACCESS

Research Access- Works Web • Works Web is the new IU Health web interface used for launching streamed applications such as Cerner via Citrix • Navigate to https: //works. iuhealth. org in the internet browser. • Sign in using the IU Health user name and password provided. • Perform Duo authentication. (IU DUO users will need a separate IU Health DUO account. ) • Click on Cerner Home Remote icon. (This may take a few moments to open. ) • Click on the Power. Chart PROD icon.

RESEARCH VIEWPOINT

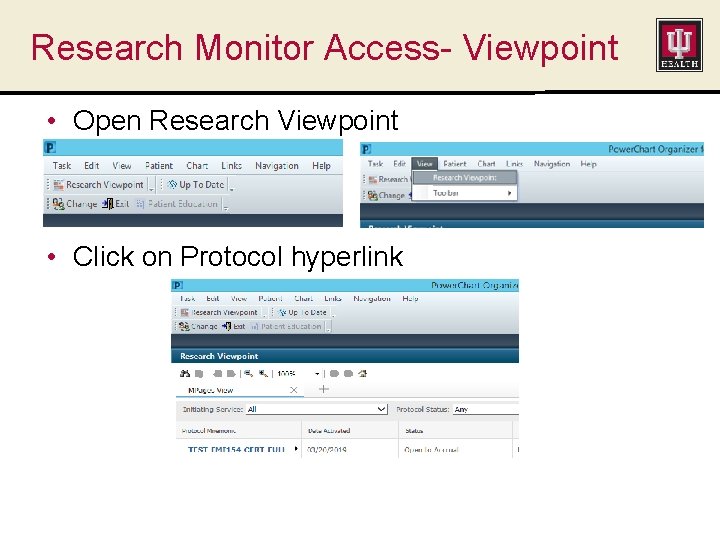
Research Monitor Access- Viewpoint • Open Research Viewpoint • Click on Protocol hyperlink

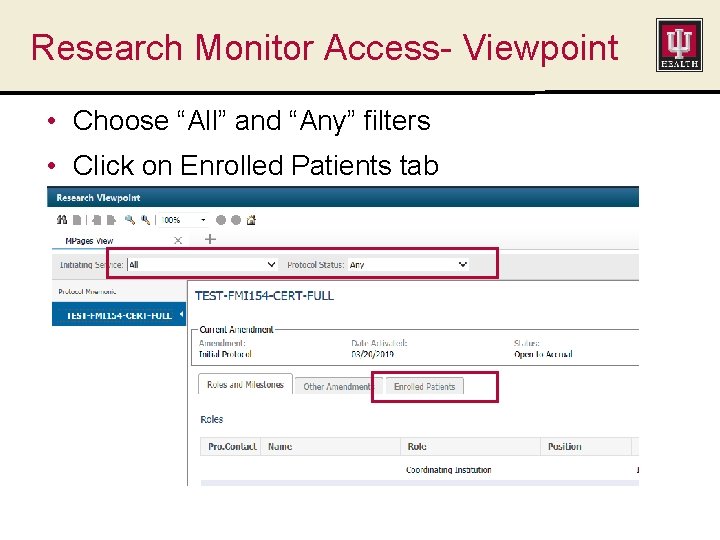
Research Monitor Access- Viewpoint • Choose “All” and “Any” filters • Click on Enrolled Patients tab

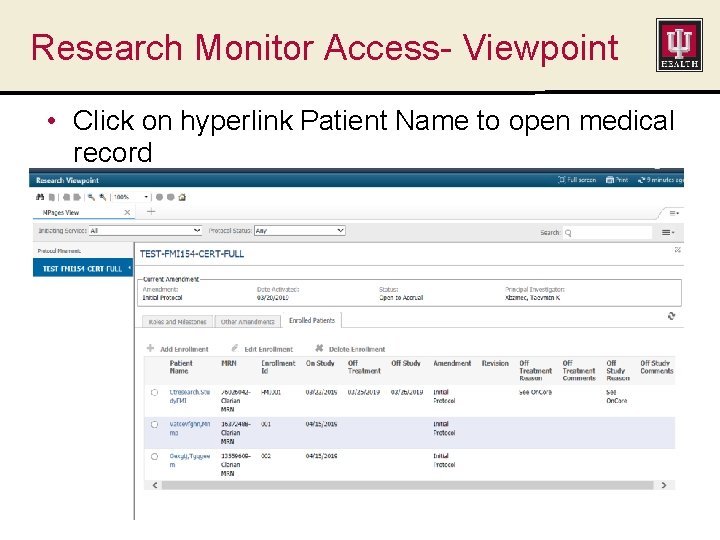
Research Monitor Access- Viewpoint • Click on hyperlink Patient Name to open medical record

Research Monitor Access- Viewpoint

WHY CAN’T MY MONITOR SEE THE SUBJECTS?

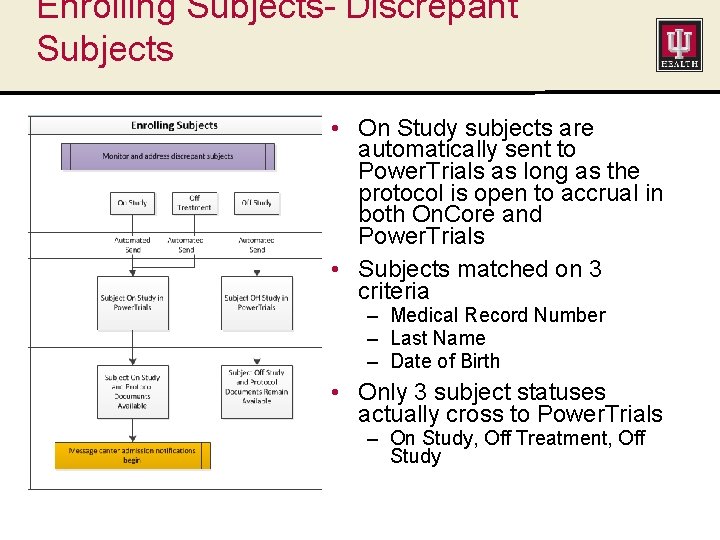
Enrolling Subjects- Discrepant Subjects • On Study subjects are automatically sent to Power. Trials as long as the protocol is open to accrual in both On. Core and Power. Trials • Subjects matched on 3 criteria – Medical Record Number – Last Name – Date of Birth • Only 3 subject statuses actually cross to Power. Trials – On Study, Off Treatment, Off Study

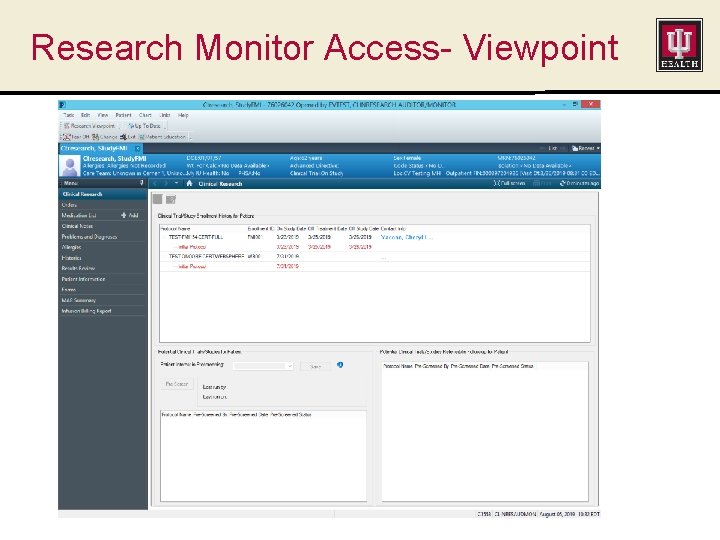
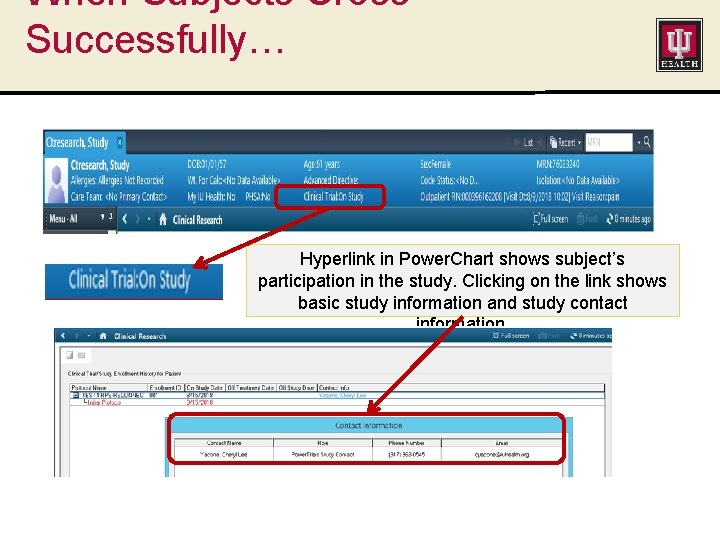
When Subjects Cross Successfully… Hyperlink in Power. Chart shows subject’s participation in the study. Clicking on the link shows basic study information and study contact information.

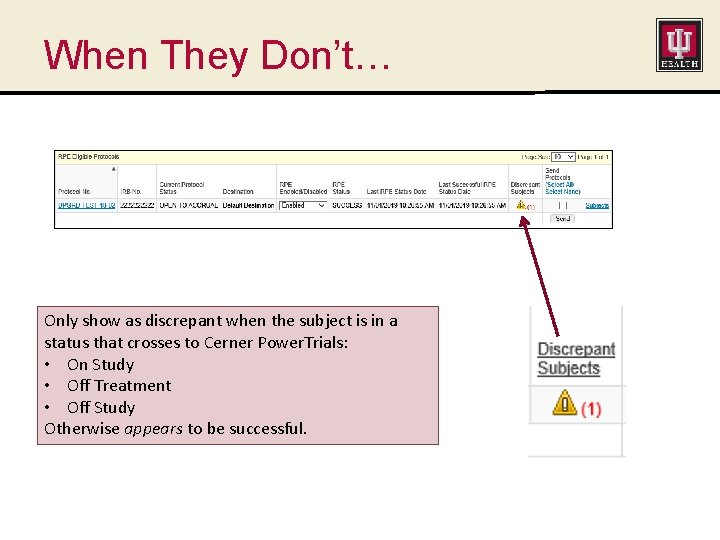
When They Don’t… Only show as discrepant when the subject is in a status that crosses to Cerner Power. Trials: • On Study • Off Treatment • Off Study Otherwise appears to be successful.

Figuring Out Why? ?

Common Subject Failure Scenarios
![Missing Consent Date Missing active start date for subject MRN number A consent Missing Consent Date Missing active start date for subject: [MRN number] • A consent](https://slidetodoc.com/presentation_image_h2/6cdaceaf031a37fdf4bc4b74e68bc704/image-17.jpg)
Missing Consent Date Missing active start date for subject: [MRN number] • A consent date has not been entered for the subject in On. Core ü Enter the consent date in On. Core; subject should cross once entered

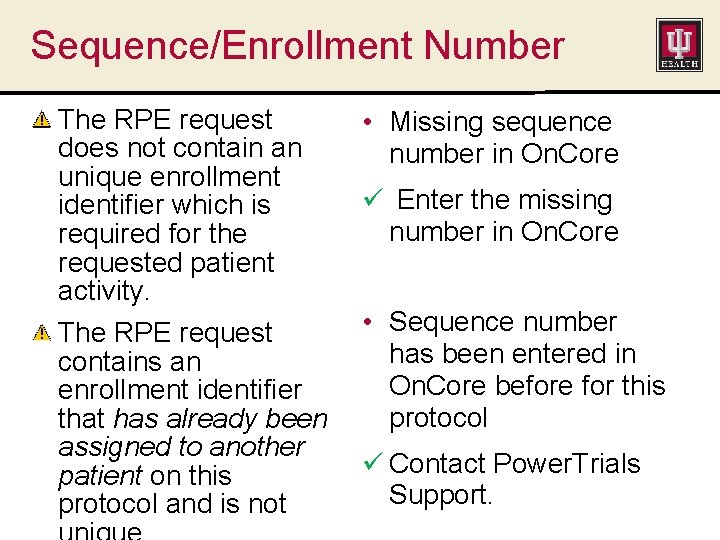
Sequence/Enrollment Number The RPE request does not contain an unique enrollment identifier which is required for the requested patient activity. The RPE request contains an enrollment identifier that has already been assigned to another patient on this protocol and is not • Missing sequence number in On. Core ü Enter the missing number in On. Core • Sequence number has been entered in On. Core before for this protocol ü Contact Power. Trials Support.

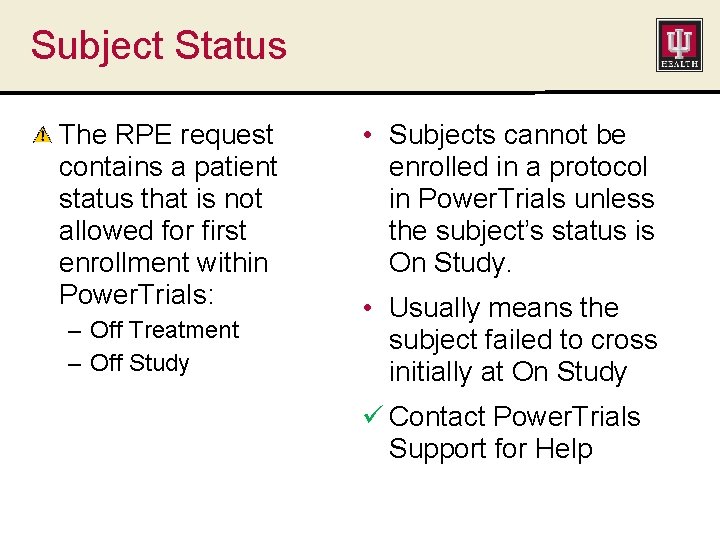
Subject Status The RPE request contains a patient status that is not allowed for first enrollment within Power. Trials: – Off Treatment – Off Study • Subjects cannot be enrolled in a protocol in Power. Trials unless the subject’s status is On Study. • Usually means the subject failed to cross initially at On Study ü Contact Power. Trials Support for Help

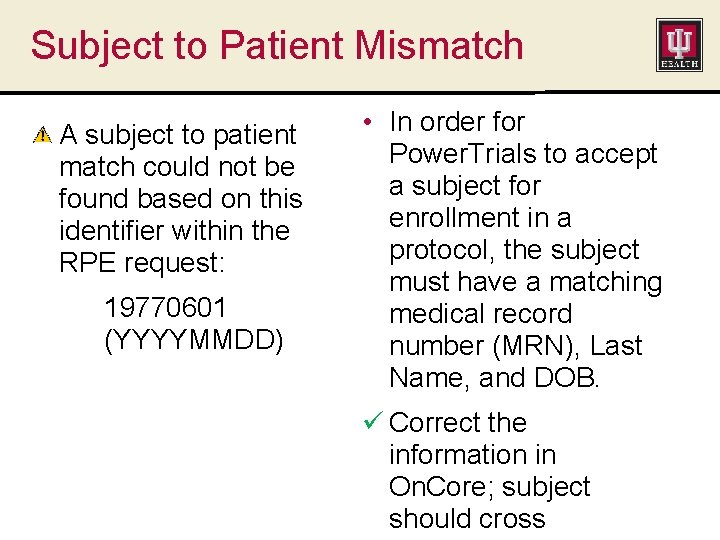
Subject to Patient Mismatch A subject to patient match could not be found based on this identifier within the RPE request: 19770601 (YYYYMMDD) • In order for Power. Trials to accept a subject for enrollment in a protocol, the subject must have a matching medical record number (MRN), Last Name, and DOB. ü Correct the information in On. Core; subject should cross

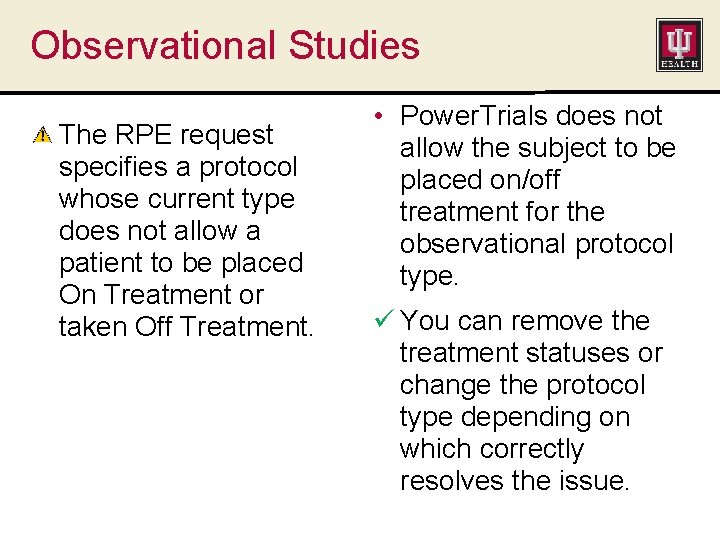
Observational Studies The RPE request specifies a protocol whose current type does not allow a patient to be placed On Treatment or taken Off Treatment. • Power. Trials does not allow the subject to be placed on/off treatment for the observational protocol type. ü You can remove the treatment statuses or change the protocol type depending on which correctly resolves the issue.

Re-Send Once the problem causing the failure has been corrected, the subject may cross to Power. Trials automatically. For some errors, it may be necessary to “push” the subject over to Power. Trials using the manual “Send All” or Send Discrepant” buttons located in the Subjects tab of the RPE Console. 1/12/2022 22

On Study/On Treatment Issue • It is possible to miss a discrepant subject who has been moved to On Treatment status closely following On Study status since On Treatment is a subject status that does not cross to Power. Trials. ü If you see that the subject On Study status is Failure but the On Treatment status says Success, contact the Power. Trials Support team for assistance.

Resources Access Support IUHealth. Research. Access@iuhealth. org Power. Trials/On. Core RPE Support Cheryl Yacone BSN, RN Sr. Clinical Analyst, IS Research Team cyacone@iuhealth. org (317) 963 -0545 On. Core Support oncore@iupui. edu (317) 278 -2600 Office of Clinical Research Website (training materials) https: //ocr. iu. edu/ 1/12/2022 24