Understanding Reaction Rate Graphs Instructions Use this slide
- Slides: 10

Understanding Reaction Rate Graphs Instructions: • Use this slide show to consider some key questions about reaction rate graphs • Click on the next slide button to see the answers and an explanation about each question

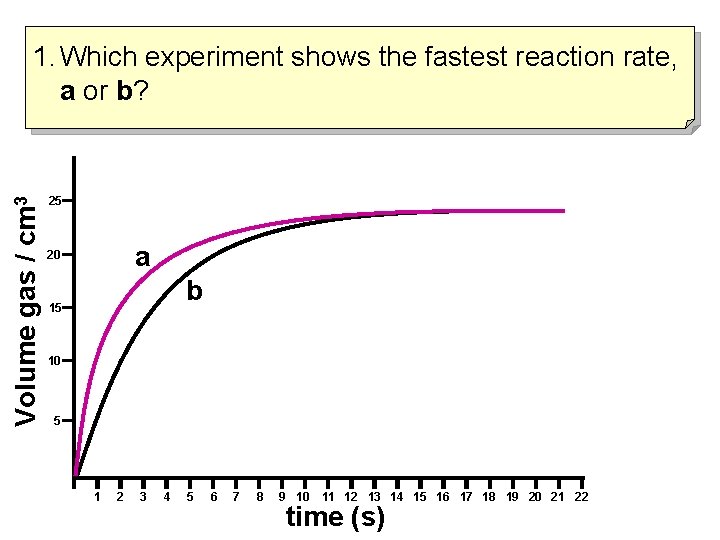
Volume gas / cm 3 1. Which experiment shows the fastest reaction rate, a or b? 25 a 20 b 15 10 5 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 time (s)

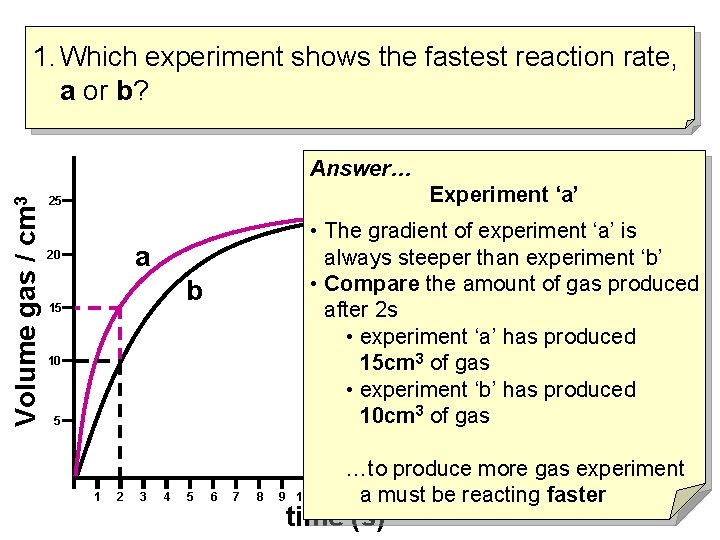
1. Which experiment shows the fastest reaction rate, a or b? Volume gas / cm 3 Answer… Experiment ‘a’ 25 • The gradient of experiment ‘a’ is always steeper than experiment ‘b’ • Compare the amount of gas produced after 2 s • experiment ‘a’ has produced 15 cm 3 of gas • experiment ‘b’ has produced 10 cm 3 of gas a 20 b 15 10 5 1 2 3 4 5 6 7 8 9 10 11 …to produce more gas experiment 12 a 13 must 14 15 be 16 17 18 19 20 faster 21 22 reacting time (s)

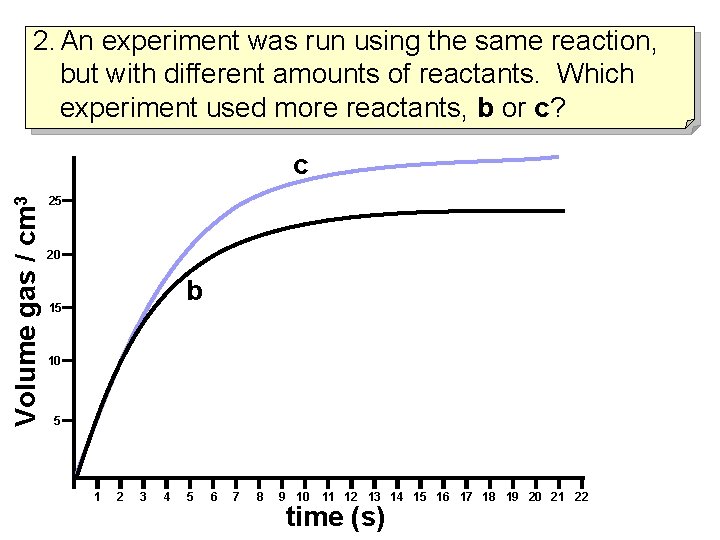
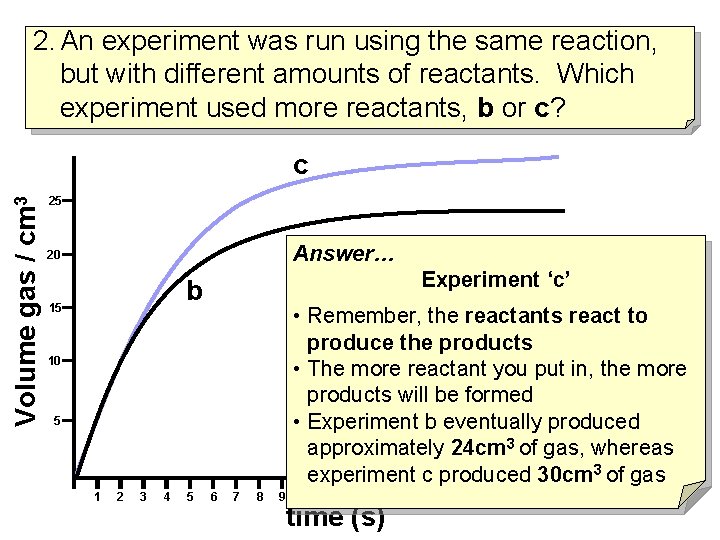
2. An experiment was run using the same reaction, but with different amounts of reactants. Which experiment used more reactants, b or c? Volume gas / cm 3 c 25 20 b 15 10 5 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 time (s)

2. An experiment was run using the same reaction, but with different amounts of reactants. Which experiment used more reactants, b or c? Volume gas / cm 3 c 25 Answer… 20 Experiment ‘c’ b 15 • Remember, the reactants react to produce the products • The more reactant you put in, the more products will be formed • Experiment b eventually produced approximately 24 cm 3 of gas, whereas experiment c produced 30 cm 3 of gas 10 5 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 time (s)

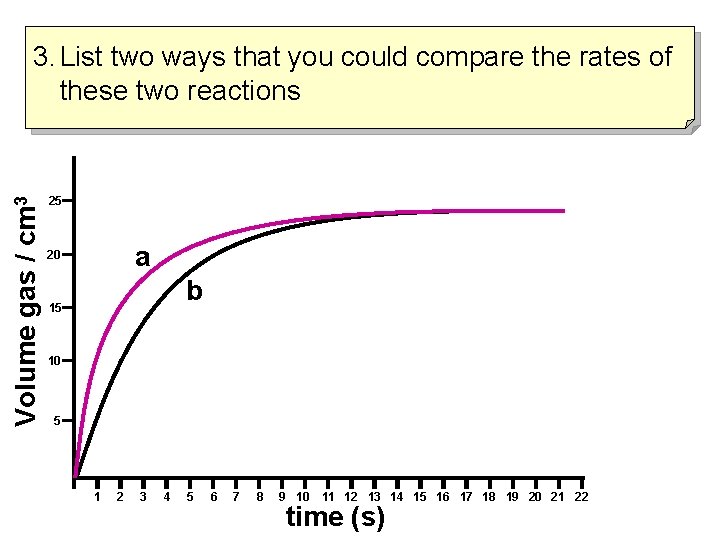
Volume gas / cm 3 3. List two ways that you could compare the rates of these two reactions 25 a 20 b 15 10 5 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 time (s)

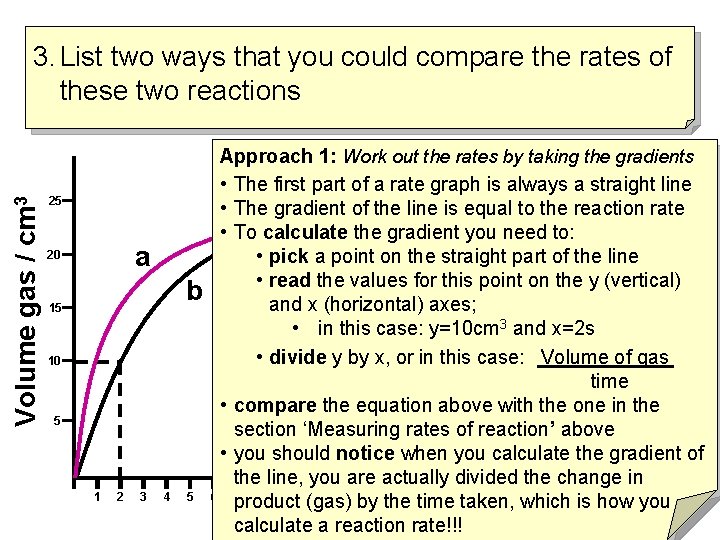
Volume gas / cm 3 3. List two ways that you could compare the rates of these two reactions 25 a 20 b 15 10 5 1 2 3 4 5 Approach 1: Work out the rates by taking the gradients • The first part of a rate graph is always a straight line • The gradient of the line is equal to the reaction rate • To calculate the gradient you need to: • pick a point on the straight part of the line • read the values for this point on the y (vertical) and x (horizontal) axes; • in this case: y=10 cm 3 and x=2 s • divide y by x, or in this case: Volume of gas time • compare the equation above with the one in the section ‘Measuring rates of reaction’ above • you should notice when you calculate the gradient of the line, you are actually divided the change in 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 product (gas) by the time taken, which is how you time (s) rate!!! calculate a reaction

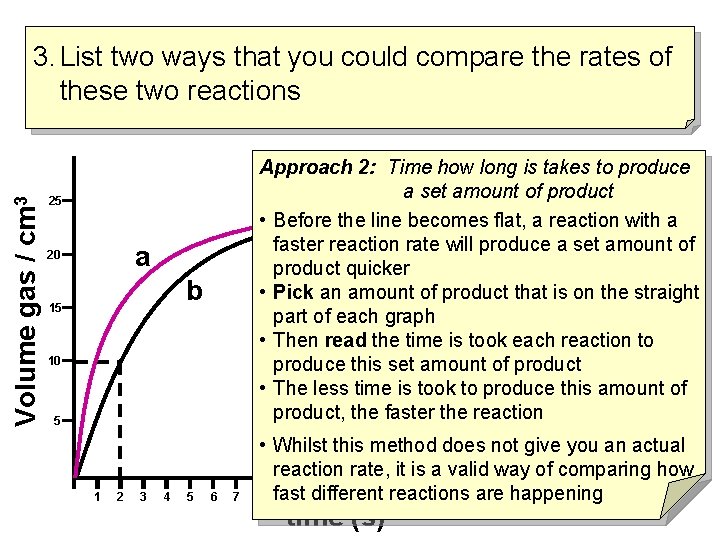
Volume gas / cm 3 3. List two ways that you could compare the rates of these two reactions Approach 2: Time how long is takes to produce a set amount of product • Before the line becomes flat, a reaction with a faster reaction rate will produce a set amount of product quicker • Pick an amount of product that is on the straight part of each graph • Then read the time is took each reaction to produce this set amount of product • The less time is took to produce this amount of product, the faster the reaction 25 a 20 b 15 10 5 1 2 3 4 5 6 7 • Whilst this method does not give you an actual reaction rate, it is a valid way of comparing how happening 8 fast 9 10 different 11 12 13 reactions 14 15 16 17 are 18 19 20 21 22 time (s)

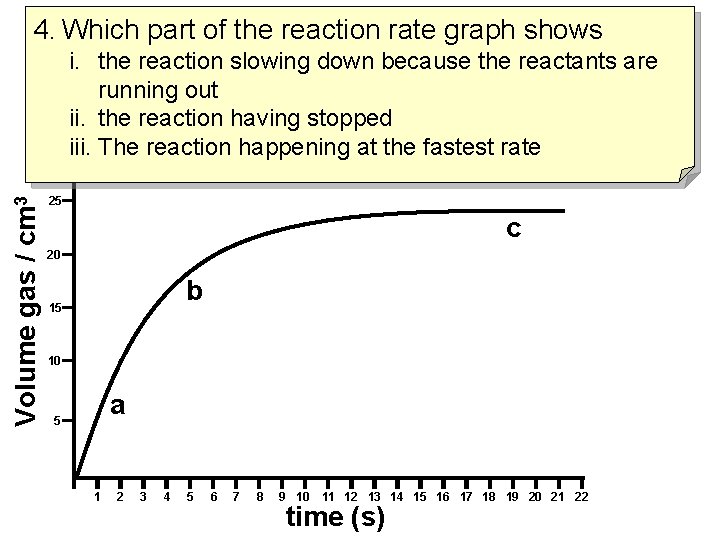
4. Which part of the reaction rate graph shows Volume gas / cm 3 i. the reaction slowing down because the reactants are running out ii. the reaction having stopped iii. The reaction happening at the fastest rate 25 c 20 b 15 10 a 5 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 time (s)

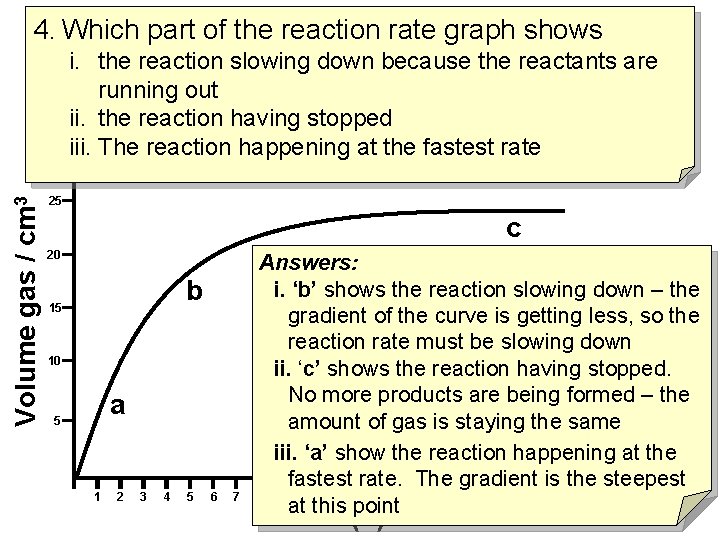
4. Which part of the reaction rate graph shows Volume gas / cm 3 i. the reaction slowing down because the reactants are running out ii. the reaction having stopped iii. The reaction happening at the fastest rate 25 c 20 b 15 10 a 5 1 2 3 4 5 6 7 Answers: i. ‘b’ shows the reaction slowing down – the gradient of the curve is getting less, so the reaction rate must be slowing down ii. ‘c’ shows the reaction having stopped. No more products are being formed – the amount of gas is staying the same iii. ‘a’ show the reaction happening at the fastest rate. The gradient is the steepest 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 at this point time (s)