Understanding Pharmaceutical Distribution This presentation is made possible

Understanding Pharmaceutical Distribution This presentation is made possible through a grant from Copyright © 2016 by the HDA Research Foundation

About this Deck • Understanding Distribution was developed as a turn-key presentation that includes an explanation of the pharmaceutical supply chain players and a discussion on the economics of distribution. • It is composed of a Power. Point module with speaking points, as well as a glossary of terms based on the 87 th Edition HDA Factbook (2016 -2017) data. • It is intended to be used by manufacturer, distributor and pharmacy presenters/trainers to “on board” new personnel, and provide data for a rich explanation of processes that support how medication moves from the manufacturer (finished product) to the customer (pharmacy/hospital/clinic). Copyright © 2016 by the HDA Research Foundation

About the Slides • The information appearing on each slide is intended to be used “as is. ” However, the deck has been developed to encourage the presenter to pick and choose the number and order of the slides to be presented. • The “notes” feature of this deck provides speaking points for each slide and allows the presenter to add or subtract concepts, thus enabling fine tuning of each presentation to suit targeted audience needs. Copyright © 2016 by the HDA Research Foundation

About this Product Legal Terms and Conditions Copyright infringement is against the law. If you believe this copy of this e-presentation you are reading infringes on the author’s copyright, please notify the publisher at: pfri@hda. org Copyright © 2016 by the HDA Research Foundation

About the Foundation The HDA Research Foundation is a 501(c)(3) non-profit charitable organization affiliated with the Healthcare Distribution Alliance (HDA). The HDA Research Foundation Mission To conduct research and disseminate information that will enhance the knowledge base, efficiency and effectiveness of the total healthcare supply chain. To provide thought leadership to further enhance the safety and security of the healthcare supply chain through future focused study and programming. Foundation Staff Contact Information Perry Fri, Executive Vice President & Chief Operating Officer. pfri@hda. org Copyright © 2016 by the HDA Research Foundation

U. S. Pharmaceutical Supply Chain Overview Manufacturer - Distributor Partnership and Operations Distributor – Provider Partnership and Operations Additional Key Supply Chain Capabilities Copyright © 2016 by the HDA Research Foundation
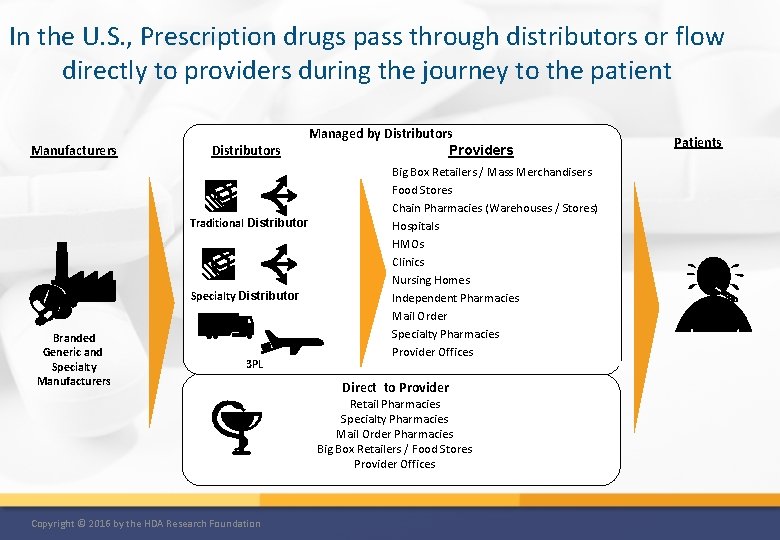
In the U. S. , Prescription drugs pass through distributors or flow directly to providers during the journey to the patient Manufacturers Distributors Managed by Distributors Providers Traditional Distributor Specialty Distributor Branded Generic and Specialty Manufacturers 3 PL Big Box Retailers / Mass Merchandisers Food Stores Chain Pharmacies (Warehouses / Stores) Hospitals HMOs Clinics Nursing Homes Independent Pharmacies Mail Order Specialty Pharmacies Provider Offices Direct to Provider Retail Pharmacies Specialty Pharmacies Mail Order Pharmacies Big Box Retailers / Food Stores Provider Offices Copyright © 2016 by the HDA Research Foundation Patients

U. S. Pharmaceuticals: Facts, Figures and Trends Copyright © 2016 by the HDA Research Foundation
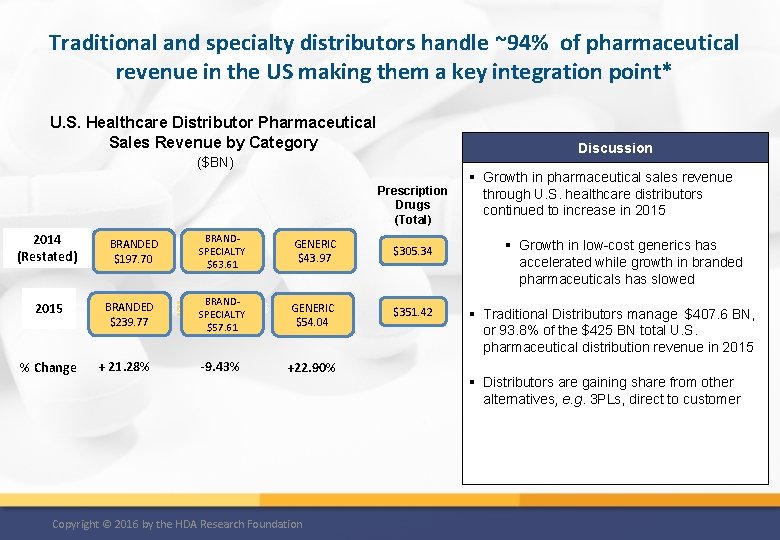
Traditional and specialty distributors handle ~94% of pharmaceutical revenue in the US making them a key integration point* U. S. Healthcare Distributor Pharmaceutical Sales Revenue by Category Discussion ($BN) Prescription Drugs (Total) 2014 (Restated) 2015 % Change BRANDED $197. 70 BRANDED $239. 77 + 21. 28% BRANDSPECIALTY $63. 61 BRAND- 32 GENERIC $43. 97 $57. 61 GENERIC $267. 32 $54. 04 -9. 43% +22. 90% $243. 26 SPECIALTY Copyright © 2016 by the HDA Research Foundation $305. 34 $351. 42 § Growth in pharmaceutical sales revenue through U. S. healthcare distributors continued to increase in 2015 § Growth in low-cost generics has accelerated while growth in branded pharmaceuticals has slowed § Traditional Distributors manage $407. 6 BN, or 93. 8% of the $425 BN total U. S. pharmaceutical distribution revenue in 2015 § Distributors are gaining share from other alternatives, e. g. 3 PLs, direct to customer
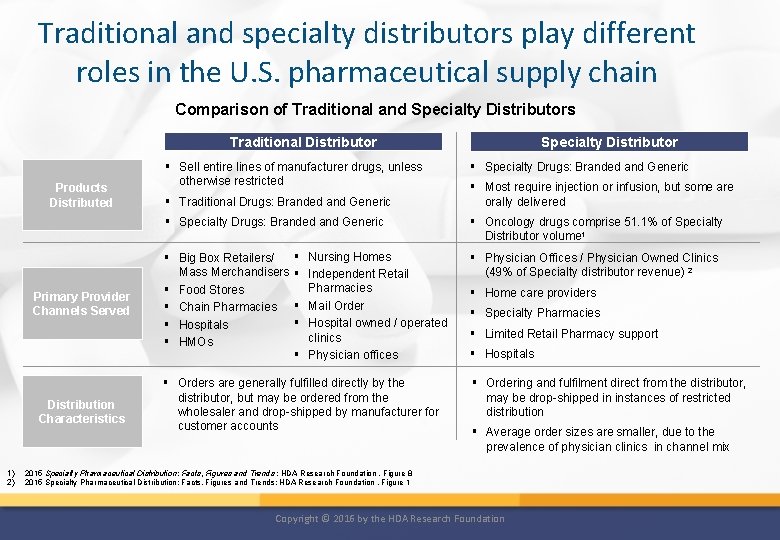
Traditional and specialty distributors play different roles in the U. S. pharmaceutical supply chain Comparison of Traditional and Specialty Distributors Traditional Distributor Products Distributed § Sell entire lines of manufacturer drugs, unless otherwise restricted § Traditional Drugs: Branded and Generic § Specialty Drugs: Branded and Generic Primary Provider Channels Served Distribution Characteristics 1) 2) § Big Box Retailers/ Mass Merchandisers § Food Stores § Chain Pharmacies § Hospitals § HMOs § Nursing Homes § Independent Retail Pharmacies § Mail Order § Hospital owned / operated clinics § Physician offices § Orders are generally fulfilled directly by the distributor, but may be ordered from the wholesaler and drop-shipped by manufacturer for customer accounts Specialty Distributor § Specialty Drugs: Branded and Generic § Most require injection or infusion, but some are orally delivered § Oncology drugs comprise 51. 1% of Specialty Distributor volume 1 § Physician Offices / Physician Owned Clinics (49% of Specialty distributor revenue) 2 § Home care providers § Specialty Pharmacies § Limited Retail Pharmacy support § Hospitals § Ordering and fulfilment direct from the distributor, may be drop-shipped in instances of restricted distribution § Average order sizes are smaller, due to the prevalence of physician clinics in channel mix 2015 Specialty Pharmaceutical Distribution: Facts, Figures and Trends ; HDA Research Foundation , Figure 8 2015 Specialty Pharmaceutical Distribution: Facts, Figures and Trends; HDA Research Foundation , Figure 1 Copyright © 2016 by the HDA Research Foundation
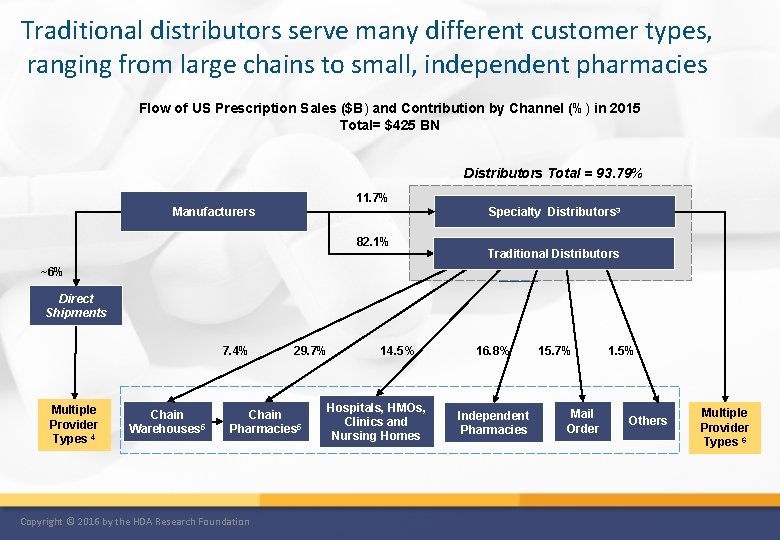
Traditional distributors serve many different customer types, ranging from large chains to small, independent pharmacies Flow of US Prescription Sales ($B) and Contribution by Channel (%) in 2015 Total= $425 BN Distributors Total = 93. 79% 11. 7% Manufacturers Specialty Distributors 3 82. 1% Traditional Distributors ~6% Direct Shipments 7. 4% Multiple Provider Types 4 Chain Warehouses 5 29. 7% Chain Pharmacies 5 Copyright © 2016 by the HDA Research Foundation 14. 5% Hospitals, HMOs, Clinics and Nursing Homes 16. 8% Independent Pharmacies 15. 7% Mail Order 1. 5% Others Multiple Provider Types 6
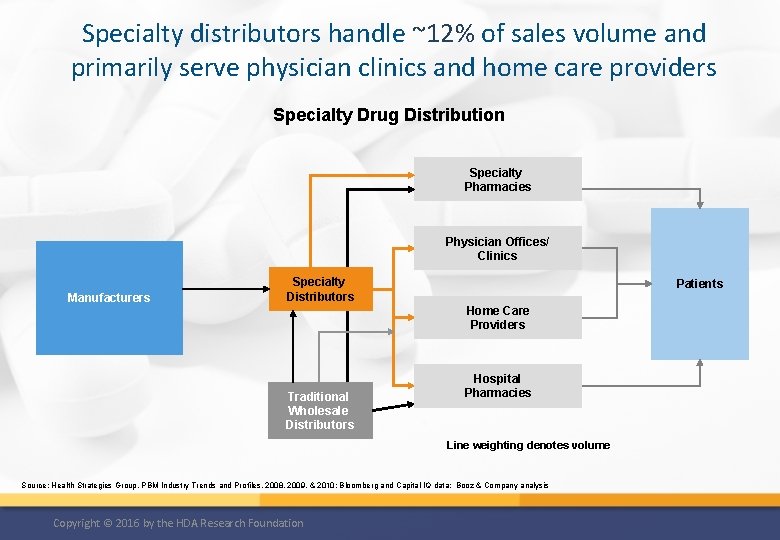
Specialty distributors handle ~12% of sales volume and primarily serve physician clinics and home care providers Specialty Drug Distribution Specialty Pharmacies Physician Offices/ Clinics Manufacturers Specialty Distributors Patients Home Care Providers Traditional Wholesale Distributors Hospital Pharmacies Line weighting denotes volume Source: Health Strategies Group, PBM Industry Trends and Profiles, 2008, 2009, & 2010; Bloomberg and Capital IQ data; Booz & Company analysis Copyright © 2016 by the HDA Research Foundation
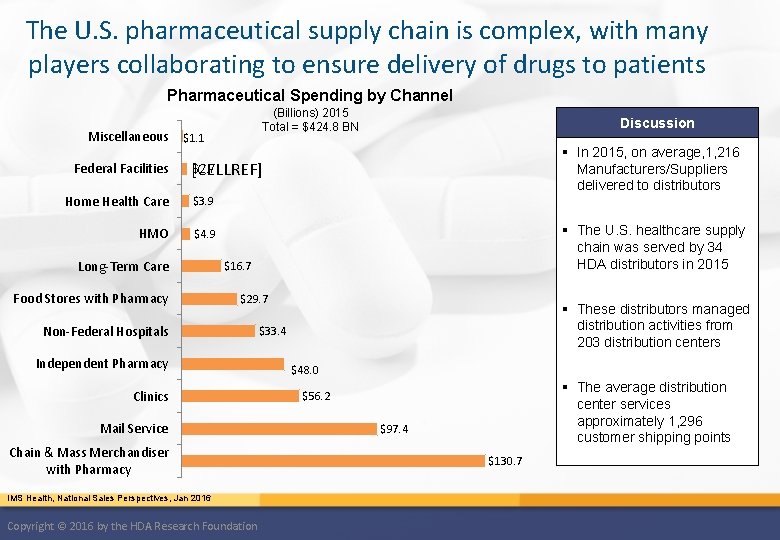
The U. S. pharmaceutical supply chain is complex, with many players collaborating to ensure delivery of drugs to patients Pharmaceutical Spending by Channel Miscellaneous Federal Facilities (Billions) 2015 Total = $424. 8 BN $1. 1 Discussion § In 2015, on average, 1, 216 Manufacturers/Suppliers delivered to distributors $2. 7 [CELLREF] Home Health Care $3. 9 HMO $4. 9 Long-Term Care Food Stores with Pharmacy § The U. S. healthcare supply chain was served by 34 HDA distributors in 2015 $16. 7 $29. 7 Non-Federal Hospitals Independent Pharmacy Clinics Mail Service Chain & Mass Merchandiser with Pharmacy IMS Health, National Sales Perspectives, Jan 2016 Copyright © 2016 by the HDA Research Foundation § These distributors managed distribution activities from 203 distribution centers $33. 4 $48. 0 § The average distribution center services approximately 1, 296 customer shipping points $56. 2 $97. 4 $130. 7

U. S. Pharmaceutical Supply Chain Overview Manufacturer - Distributor Partnership and Operations Distributor – Provider Partnership and Operations Additional Key Supply Chain Capabilities Copyright © 2016 by the HDA Research Foundation
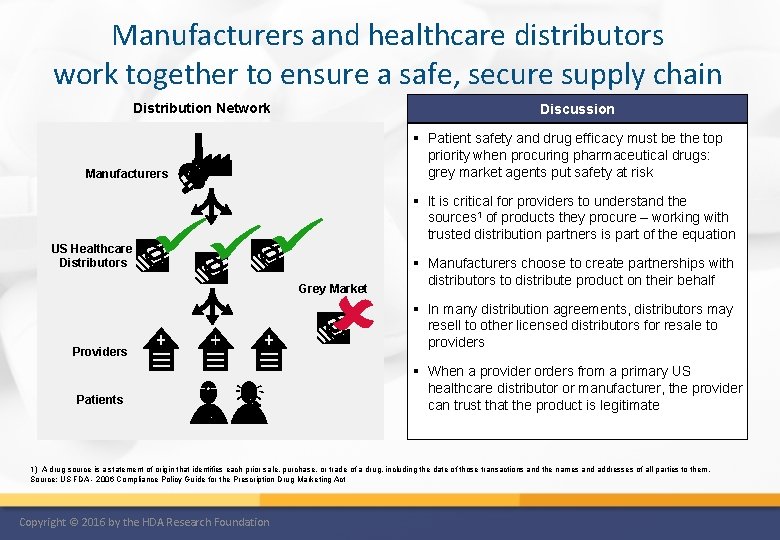
Manufacturers and healthcare distributors work together to ensure a safe, secure supply chain Distribution Network Discussion § Patient safety and drug efficacy must be the top priority when procuring pharmaceutical drugs: grey market agents put safety at risk Manufacturers § It is critical for providers to understand the sources 1 of products they procure – working with trusted distribution partners is part of the equation US Healthcare Distributors Grey Market Providers Patients § Manufacturers choose to create partnerships with distributors to distribute product on their behalf § In many distribution agreements, distributors may resell to other licensed distributors for resale to providers § When a provider orders from a primary US healthcare distributor or manufacturer, the provider can trust that the product is legitimate 1) A drug source is a statement of origin that identifies each prior sale, purchase, or trade of a drug, including the date of those transactions and the names and addresses of all parties to them. Source: US FDA - 2006 Compliance Policy Guide for the Prescription Drug Marketing Act Copyright © 2016 by the HDA Research Foundation

Manufacturers may utilize a wide array of services provided by their distribution partners Array of Distributor Services Manufacturers § Adherence management programs § Ownership of credit risk (receivables) § Business continuity risk management § Packaging and re-packaging services § Chargeback administration § Product compliance support services § Customer access and knowledge § Promotional material distribution § Data services: basic; e. g. sales, inventory, returns § Data services: enhanced; e. g. clinical performance § Provide 3 rd party support services (contract review, reimbursement support) § Disaster preparedness § REMS support § Support compliance with federal and state regulations § Reverse logistics / returns § Inventory management § Security § New product launch support § Special handling services § Order management and fulfilment § Suspicious order monitoring § Recalls § Web based portal solutions Source: Booz & Company interviews with distributors and manufacturers Note: Services listed are not exhaustive of all services provided Copyright © 2016 by the HDA Research Foundation

The pharma distribution compensation model has evolved from a volatile Buy and Hold model to the more stable Fee For Service Specialty Pharmaceutical Distribution Compensation Model… Fee For Service Compensation Model § Distributor buys the product from manufacturer, resells to provider § Manufacturer pays the distributor a Fee for Service (FFS) for an agreed upon set of services § In many cases, price appreciation gains for inventory held at the distributor may be “clawed back” to the manufacturer Today: Benefits of the Fee For Service Model § FFS agreements are the standard for branded drugs § Benefits include – Lower inventory / carrying costs – Greater supply chain transparency – Reduced volatility in revenues – Reduced volatility in the supply chain § Improved industry collaboration by enabling manufacturers to better understand distributor impact and value Copyright © 2016 by the HDA Research Foundation
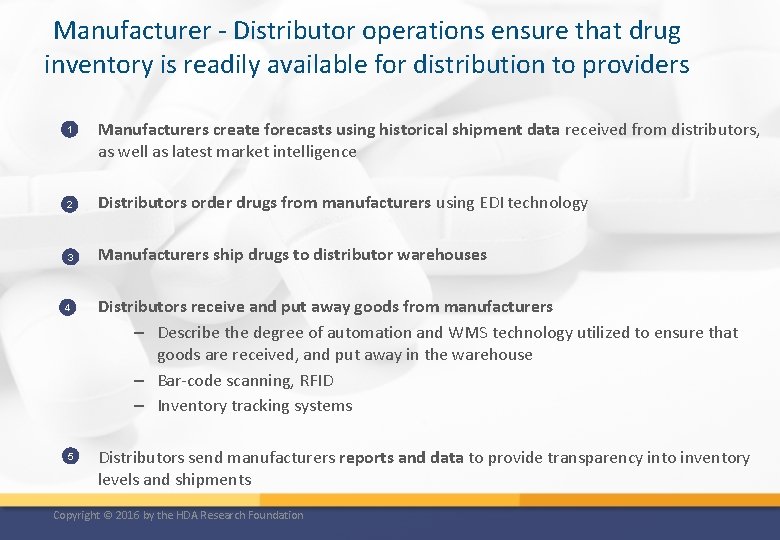
Manufacturer - Distributor operations ensure that drug inventory is readily available for distribution to providers 1 Manufacturers create forecasts using historical shipment data received from distributors, as well as latest market intelligence 2 Distributors order drugs from manufacturers using EDI technology 3 Manufacturers ship drugs to distributor warehouses 4 5 Distributors receive and put away goods from manufacturers – Describe the degree of automation and WMS technology utilized to ensure that goods are received, and put away in the warehouse – Bar-code scanning, RFID – Inventory tracking systems Distributors send manufacturers reports and data to provide transparency into inventory levels and shipments Copyright © 2016 by the HDA Research Foundation
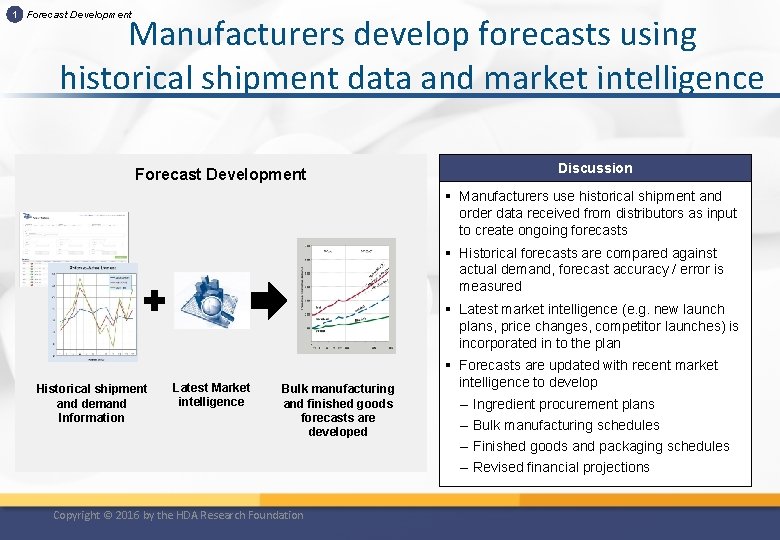
1 Forecast Development Manufacturers develop forecasts using historical shipment data and market intelligence Forecast Development Discussion § Manufacturers use historical shipment and order data received from distributors as input to create ongoing forecasts § Historical forecasts are compared against actual demand, forecast accuracy / error is measured § Latest market intelligence (e. g. new launch plans, price changes, competitor launches) is incorporated in to the plan Historical shipment and demand Information Latest Market intelligence Bulk manufacturing and finished goods forecasts are developed Copyright © 2016 by the HDA Research Foundation § Forecasts are updated with recent market intelligence to develop – Ingredient procurement plans – Bulk manufacturing schedules – Finished goods and packaging schedules – Revised financial projections
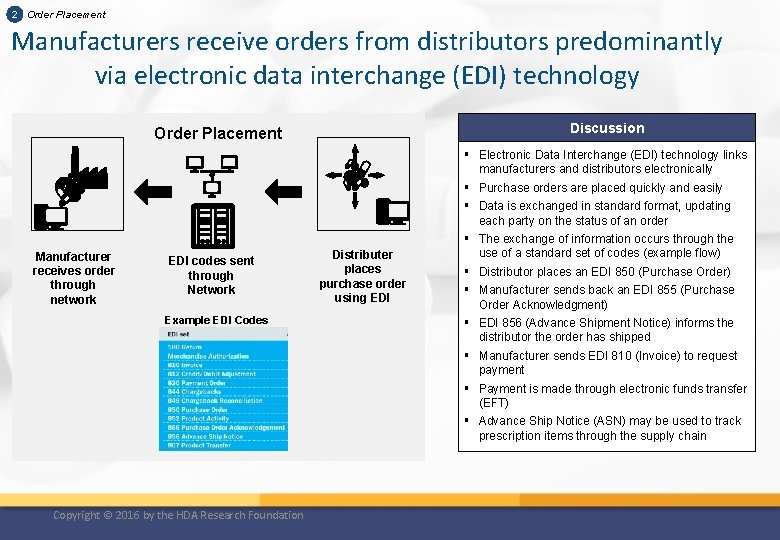
2 Order Placement Manufacturers receive orders from distributors predominantly via electronic data interchange (EDI) technology Discussion Order Placement Manufacturer receives order through network EDI codes sent through Network Example EDI Codes Copyright © 2016 by the HDA Research Foundation Distributer places purchase order using EDI § Electronic Data Interchange (EDI) technology links manufacturers and distributors electronically § Purchase orders are placed quickly and easily § Data is exchanged in standard format, updating each party on the status of an order § The exchange of information occurs through the use of a standard set of codes (example flow) § Distributor places an EDI 850 (Purchase Order) § Manufacturer sends back an EDI 855 (Purchase Order Acknowledgment) § EDI 856 (Advance Shipment Notice) informs the distributor the order has shipped § Manufacturer sends EDI 810 (Invoice) to request payment § Payment is made through electronic funds transfer (EFT) § Advance Ship Notice (ASN) may be used to track prescription items through the supply chain
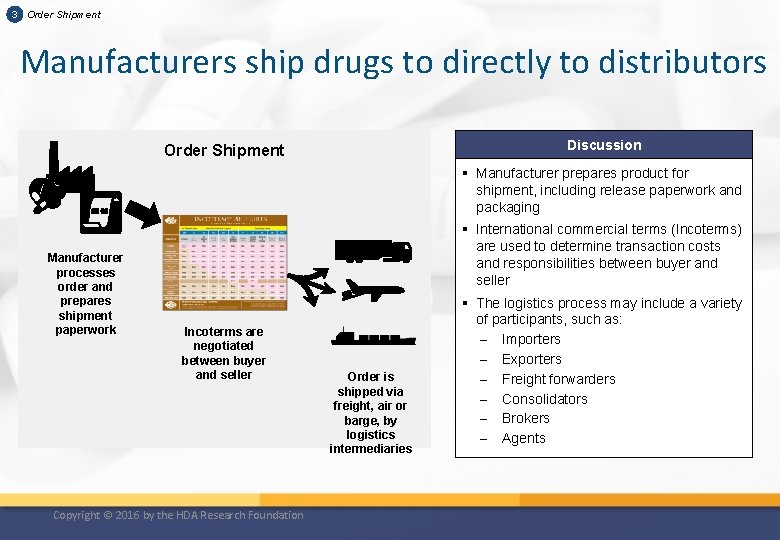
3 Order Shipment Manufacturers ship drugs to directly to distributors Discussion Order Shipment § Manufacturer prepares product for shipment, including release paperwork and packaging Manufacturer processes order and prepares shipment paperwork § International commercial terms (Incoterms) are used to determine transaction costs and responsibilities between buyer and seller Incoterms are negotiated between buyer and seller Copyright © 2016 by the HDA Research Foundation Order is shipped via freight, air or barge, by logistics intermediaries § The logistics process may include a variety of participants, such as: ‒ Importers ‒ Exporters ‒ Freight forwarders ‒ Consolidators ‒ Brokers ‒ Agents
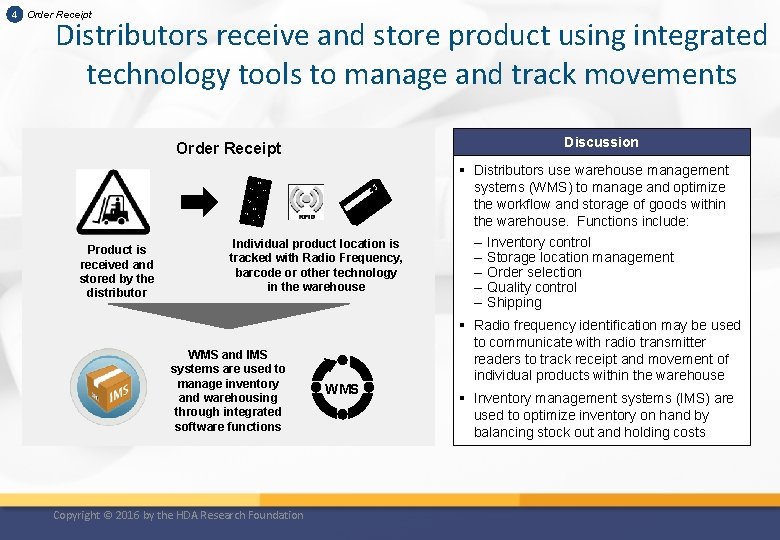
4 Order Receipt Distributors receive and store product using integrated technology tools to manage and track movements Discussion Order Receipt Product is received and stored by the distributor Individual product location is tracked with Radio Frequency, barcode or other technology in the warehouse WMS and IMS systems are used to manage inventory and warehousing through integrated software functions Copyright © 2016 by the HDA Research Foundation WMS § Distributors use warehouse management systems (WMS) to manage and optimize the workflow and storage of goods within the warehouse. Functions include: – Inventory control – Storage location management – Order selection – Quality control – Shipping § Radio frequency identification may be used to communicate with radio transmitter readers to track receipt and movement of individual products within the warehouse § Inventory management systems (IMS) are used to optimize inventory on hand by balancing stock out and holding costs
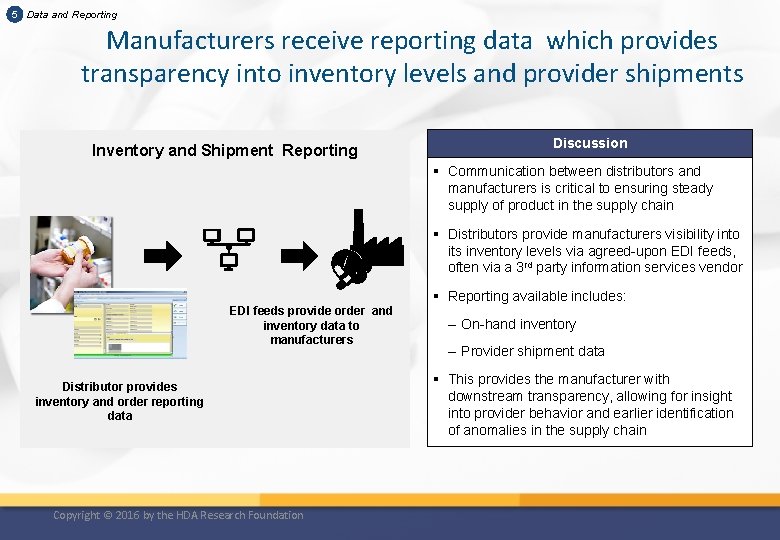
5 Data and Reporting Manufacturers receive reporting data which provides transparency into inventory levels and provider shipments Inventory and Shipment Reporting Discussion § Communication between distributors and manufacturers is critical to ensuring steady supply of product in the supply chain § Distributors provide manufacturers visibility into its inventory levels via agreed-upon EDI feeds, often via a 3 rd party information services vendor § Reporting available includes: EDI feeds provide order and inventory data to manufacturers Distributor provides inventory and order reporting data Copyright © 2016 by the HDA Research Foundation – On-hand inventory – Provider shipment data § This provides the manufacturer with downstream transparency, allowing for insight into provider behavior and earlier identification of anomalies in the supply chain

U. S. Pharmaceutical Supply Chain Overview Manufacturer - Distributor Partnership and Operations Distributor – Provider Partnership and Operations Additional Key Supply Chain Capabilities

Providers elect to work with a primary distribution partner who ensures safe, timely supply and may provide many other services Services Offered by Distributors to Providers § Adherence / compliance programs and tracking § Pharmacy management systems and services § Business continuity risk management § Planogramming services § Centralized information and improved communication flow § Product compliance support services § Chargeback administration § Provide 3 rd party support services (contract review, reimbursement support) § Disaster preparedness § REMS support § Support compliance with federal and state regulations § Rack-Jobbing (in-store replenishment) § Financial management / access to credit § Reverse logistics / Returns § Inventory management § Recalls § Order management and fulfilment § Security § Payer economic modelling and negotiation support § Special handling services Source: Booz & Company interviews with distributors, manufacturers, retail pharmacies Note: Services listed are not exhaustive of all services provided Copyright © 2016 by the HDA Research Foundation § Reconciliation services
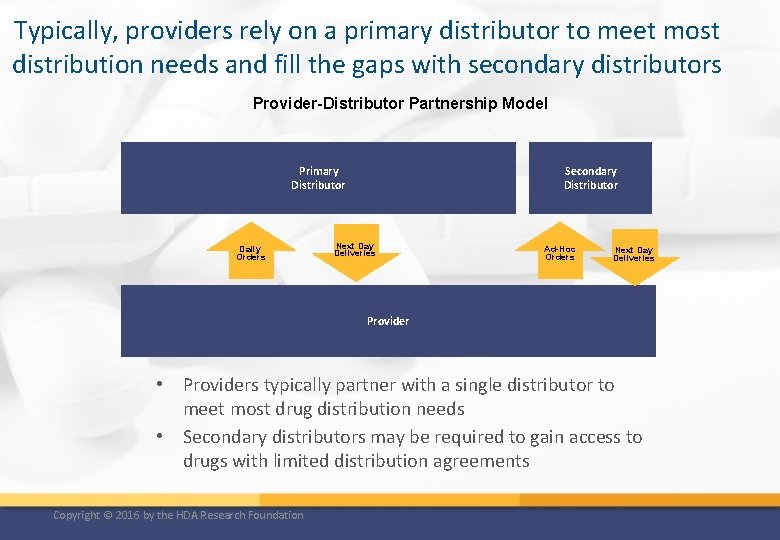
Typically, providers rely on a primary distributor to meet most distribution needs and fill the gaps with secondary distributors Provider-Distributor Partnership Model Primary Distributor Daily Orders Secondary Distributor Next Day Deliveries Ad-Hoc Orders Next Day Deliveries Provider • Providers typically partner with a single distributor to meet most drug distribution needs • Secondary distributors may be required to gain access to drugs with limited distribution agreements Copyright © 2016 by the HDA Research Foundation

Distributors review provider licenses, registration, and other information prior to providing prescription drug products Provider Review Discussion § Prior to distributing drugs to a provider, the distributor reviews provider credentials § Distributors solicits providers credentials Provider wishes to obtain distribution services Distributor solicits provider credentials, may perform additional due-diligence Copyright © 2016 by the HDA Research Foundation § Prior to shipping certain controlled substances (e. g. Opioids), distributors may conduct further review, which may include: – Visiting the provider location – Examining historical dispensing data from the provider – Evaluating provider expected order quantities for controlled substances – Monitor provider orders on an ongoing basis

The provider order fulfillment process is highly efficient, ensuring safe, secure delivery of drugs in a timely manner A Day in the Life of a Provider Pharmaceutical Order 1 Provider places an order with their distribution partner (EDI, online ordering, phone or fax) 2 Distributor receives the order, which is routed to the distribution center 3 Sophisticated WMS systems are used to enable the pick-pack-ship operations to run smoothly and efficiently (examples of systems, and capabilities of sophisticated WMS systems) 4 Orders are picked using different technology based on inventory velocity and product handling requirements 5 Order is packed and shipped via Common Courier or a Delivery Truck, depending on the size of the order 6 Order is received at the provider, in some cases, as early as the same day of placement Copyright © 2016 by the HDA Research Foundation
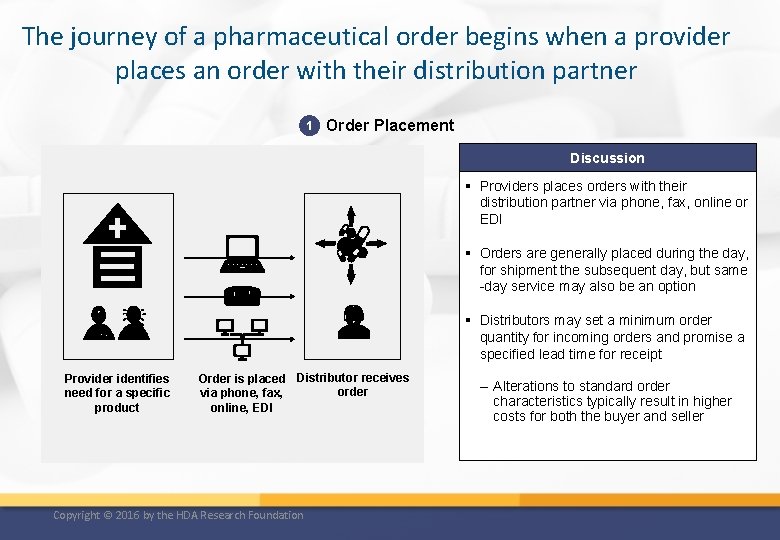
The journey of a pharmaceutical order begins when a provider places an order with their distribution partner 1 Order Placement Discussion § Providers places orders with their distribution partner via phone, fax, online or EDI § Orders are generally placed during the day, for shipment the subsequent day, but same -day service may also be an option § Distributors may set a minimum order quantity for incoming orders and promise a specified lead time for receipt Provider identifies need for a specific product Order is placed Distributor receives order via phone, fax, online, EDI Copyright © 2016 by the HDA Research Foundation – Alterations to standard order characteristics typically result in higher costs for both the buyer and seller
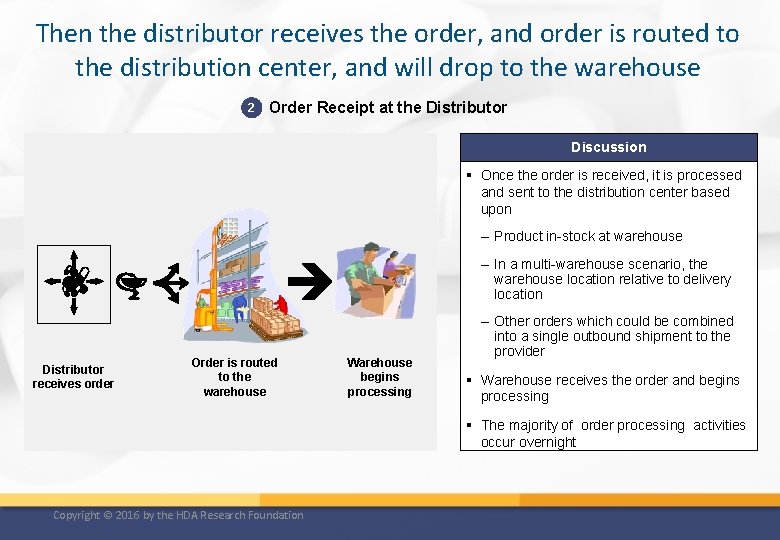
Then the distributor receives the order, and order is routed to the distribution center, and will drop to the warehouse 2 Order Receipt at the Distributor Discussion § Once the order is received, it is processed and sent to the distribution center based upon – Product in-stock at warehouse – In a multi-warehouse scenario, the warehouse location relative to delivery location Distributor receives order Order is routed to the warehouse Warehouse begins processing – Other orders which could be combined into a single outbound shipment to the provider § Warehouse receives the order and begins processing § The majority of order processing activities occur overnight Copyright © 2016 by the HDA Research Foundation
Sophisticated WMS systems enable distributor pick-pack-ship operations to run smoothly and efficiently 3 Use of WMS Systems Discussion § At the warehouse, the order is received by the warehouse management system (WMS) WMS receives the order and generates a pickpack-ship plan Copyright © 2016 by the HDA Research Foundation § WMS performs functions that enable efficient order processing – Receiving – Inventory control – Order entry – Order selection and visibility – Quality control – Storage location management – Automated replenishment – Shipping
Order is picked using automated or manual technology based on inventory velocity and product handling requirements 4 Order Picking Discussion § WMS provides staff with efficient picking sequences, product location, and inventory levels § Automated technologies such as radio frequency (RF) wireless devices, carousels, A-Frames and sortation systems may be used to help staff efficiently pick products § WMS automatically creates replenishment orders to manufacturers at specified levels § Shipping plans are generated and order status is updated through WMS Automatic identification and communication technologies enable efficient pick-pack Product may also be picked manually Copyright © 2016 by the HDA Research Foundation § Controls are in place to ensure that the right product and quantities are included in the shipment
When the full order has been picked and packed, it will be sent to the provider 5 Order Packing and Shipping Discussion § Warehouse prepares order for shipment, including required paperwork and packaging § Orders will be shipped in different modes depending on order size and service level agreements including – Small parcel shipments – Courier delivery – Less than truckload (LTL) or full truckload (FTL) shipments Warehouse prepares order for shipment Order is shipped via most appropriate transportation method Copyright © 2016 by the HDA Research Foundation § Orders for different customers may also be combined in one shipment to minimize costs
Order is received at the provider, as early as the same day of placement depending on service level agreements 6 Order Receipt by Provider Discussion § Typically, orders are placed in one day, then shipped and received the next § Depending on urgency or service level agreements, product may be ordered and delivered on the same day § Provider receives the product as well as administrative tracing information. § Provider stores drugs in appropriate conditions upon receipt (e. g. room temperature, 2 -8°C) Order is delivered to provider Provider receives order with appropriate paperwork Copyright © 2016 by the HDA Research Foundation
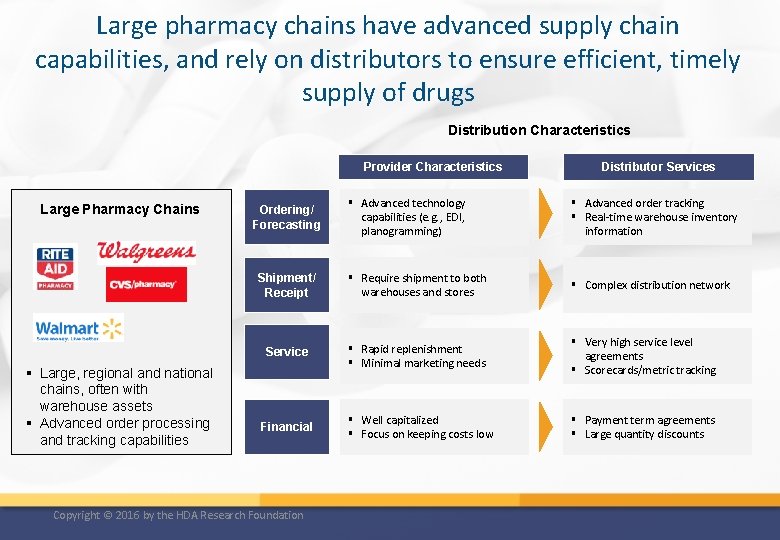
Large pharmacy chains have advanced supply chain capabilities, and rely on distributors to ensure efficient, timely supply of drugs Distribution Characteristics Provider Characteristics Large Pharmacy Chains § Large, regional and national chains, often with warehouse assets § Advanced order processing and tracking capabilities Distributor Services § Advanced technology capabilities (e. g. , EDI, planogramming) § Advanced order tracking § Real-time warehouse inventory information Shipment/ Receipt § Require shipment to both warehouses and stores § Complex distribution network Service § Rapid replenishment § Minimal marketing needs § Very high service level agreements § Scorecards/metric tracking § Well capitalized § Focus on keeping costs low § Payment term agreements § Large quantity discounts Ordering/ Forecasting Financial Copyright © 2016 by the HDA Research Foundation
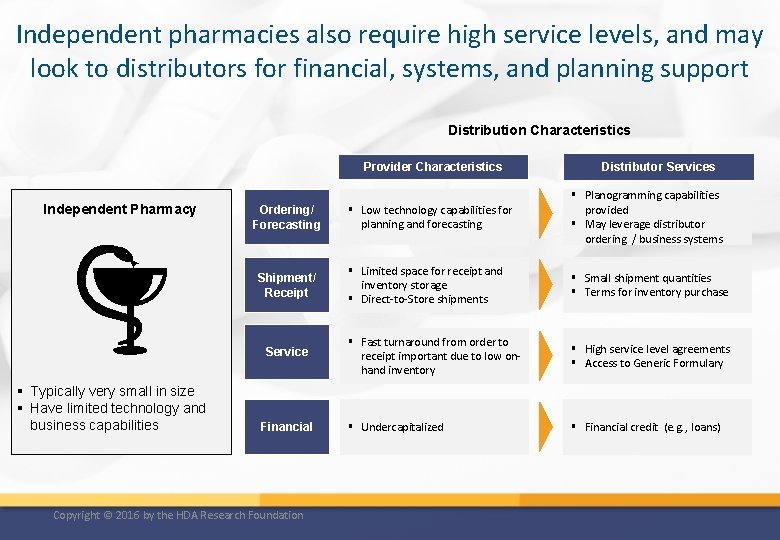
Independent pharmacies also require high service levels, and may look to distributors for financial, systems, and planning support Distribution Characteristics Provider Characteristics Independent Pharmacy Ordering/ Forecasting Shipment/ Receipt Service § Typically very small in size § Have limited technology and business capabilities Financial Copyright © 2016 by the HDA Research Foundation Distributor Services § Low technology capabilities for planning and forecasting § Planogramming capabilities provided § May leverage distributor ordering / business systems § Limited space for receipt and inventory storage § Direct-to-Store shipments § Small shipment quantities § Terms for inventory purchase § Fast turnaround from order to receipt important due to low onhand inventory § High service level agreements § Access to Generic Formulary § Undercapitalized § Financial credit (e. g. , loans)
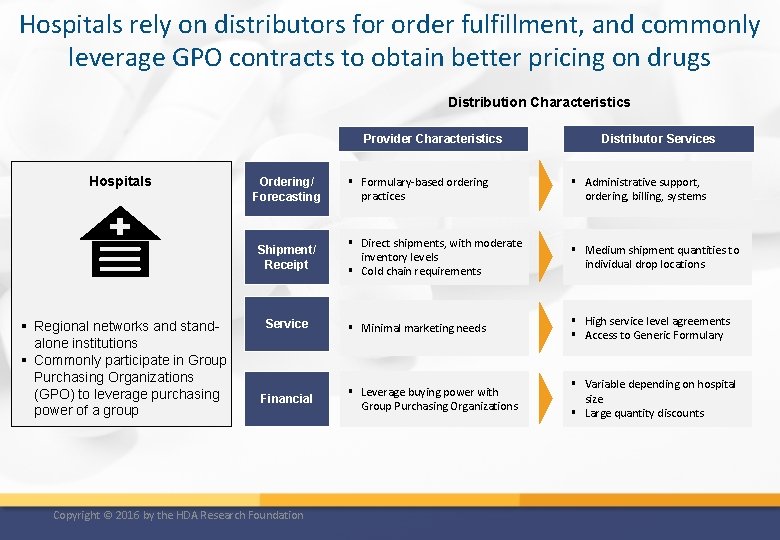
Hospitals rely on distributors for order fulfillment, and commonly leverage GPO contracts to obtain better pricing on drugs Distribution Characteristics Provider Characteristics Hospitals Ordering/ Forecasting Shipment/ Receipt § Regional networks and standalone institutions § Commonly participate in Group Purchasing Organizations (GPO) to leverage purchasing power of a group Service Financial Copyright © 2016 by the HDA Research Foundation Distributor Services § Formulary-based ordering practices § Administrative support, ordering, billing, systems § Direct shipments, with moderate inventory levels § Cold chain requirements § Medium shipment quantities to individual drop locations § Minimal marketing needs § High service level agreements § Access to Generic Formulary § Leverage buying power with Group Purchasing Organizations § Variable depending on hospital size § Large quantity discounts
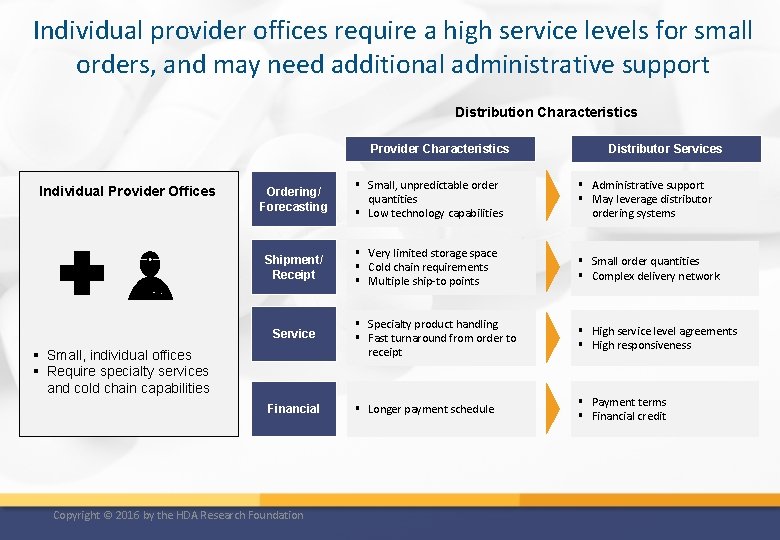
Individual provider offices require a high service levels for small orders, and may need additional administrative support Distribution Characteristics Provider Characteristics Individual Provider Offices Distributor Services Ordering/ Forecasting § Small, unpredictable order quantities § Low technology capabilities § Administrative support § May leverage distributor ordering systems Shipment/ Receipt § Very limited storage space § Cold chain requirements § Multiple ship-to points § Small order quantities § Complex delivery network § Specialty product handling § Fast turnaround from order to receipt § High service level agreements § High responsiveness § Longer payment schedule § Payment terms § Financial credit Service § Small, individual offices § Require specialty services and cold chain capabilities Financial Copyright © 2016 by the HDA Research Foundation

U. S. Pharmaceutical Supply Chain Overview Manufacturer - Distributor Partnership and Operations Distributor – Provider Partnership and Operations Additional Key Supply Chain Capabilities Copyright © 2016 by the HDA Research Foundation

Additional supply chain capabilities and intricacies exist, including the following… 1 2 3 4 New Product Introduction support – partnering with manufacturers to ensure efficient market access to providers, ensuring access to drugs around product launch Special handling capabilities – Unique capabilities are required to ensure flawless management and delivery of drugs requiring cold chain handling and other special handling needs Reverse Logistics, Returns and Recall support Financial Support – in particular, providing credit terms to providers, which insulates manufacturers from credit risk Copyright © 2016 by the HDA Research Foundation

The pharma industry is constantly evolving, and has faced significant economic pressures as well as structural challenges Challenges in the Pharmaceutical Market Additional Pressure from Economic Crisis 1 Existing Structural Challenges § Limited new product launches § Increasingly restrictive approval and reimbursement requirements § Growth shifting to lower price point emerging markets § Customer consolidation increasing customer buying power (e. g. retail pharmacy) § New and increased focused on regulations § Drug Quality and Security Act (DQSA) Industry Considerations § Shrinking revenue for public health care budgets intensifies cost containment measures – Drug price reduction – Generic substitution – Demand for proof of value § Generics and branded categories are growing § Consumers have less to spend and unemployment has decreased the population covered with insurance § Consolidation of retailers and manufacturers continues; merging players seek to capitalize on both revenue and cost synergies § Independent pharmacies struggle financially Copyright © 2016 by the HDA Research Foundation § Patent cliff revenue pressure drives manufacturers to seek cost reduction opportunities and clear ROI on outsourced services § The regulatory environment affects the cost of doing business, yet also provides opportunities to offer new, value added services
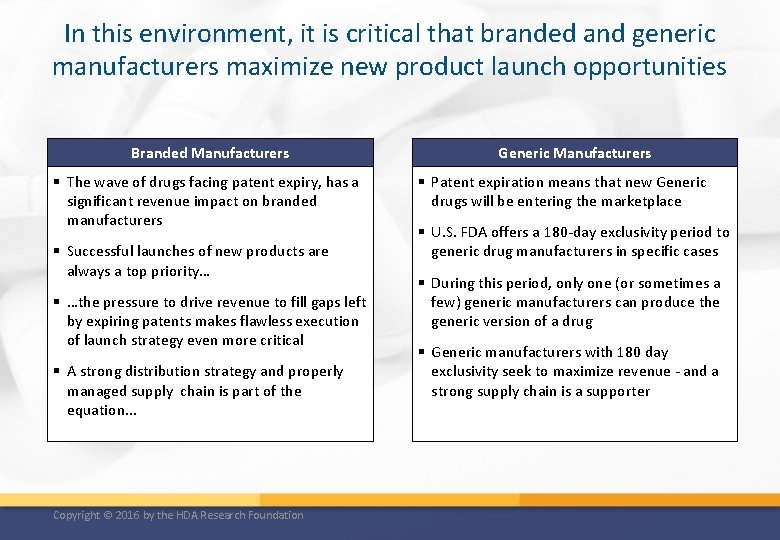
In this environment, it is critical that branded and generic manufacturers maximize new product launch opportunities Branded Manufacturers § The wave of drugs facing patent expiry, has a significant revenue impact on branded manufacturers § Successful launches of new products are always a top priority… § …the pressure to drive revenue to fill gaps left by expiring patents makes flawless execution of launch strategy even more critical § A strong distribution strategy and properly managed supply chain is part of the equation. . . Copyright © 2016 by the HDA Research Foundation Generic Manufacturers § Patent expiration means that new Generic drugs will be entering the marketplace § U. S. FDA offers a 180 -day exclusivity period to generic drug manufacturers in specific cases § During this period, only one (or sometimes a few) generic manufacturers can produce the generic version of a drug § Generic manufacturers with 180 day exclusivity seek to maximize revenue - and a strong supply chain is a supporter

A successful brand or generic launch requires a well-designed distribution strategy, designed to meet provider and market needs New Product Launch: Key Success Factors - Distribution • Provider access – Drugs should be easy for the appropriate providers to obtain. For branded manufacturers a more open distribution strategy helps to ensures that products will be available from a provider’s preferred distribution partner • Pre-Launch Inventory Planning and Staging – Prior to launch, inventories must be prepositioned in the supply chain to help ensure ready access to providers • Service Levels – Drug must be available to providers in a timely manner – delays in access can affect patient care and market share, particularly when competitor substitutes exist • Regulatory Compliance – Distribution plans must comply with regulatory mandates from the DEA, as well as any REMS ETASU requirements Copyright © 2016 by the HDA Research Foundation

Pharma manufacturers must carefully consider the appropriate supply chain strategy to reach the market New Product Launch: Supply Chain Strategy Choices Open Distribution § Open distribution through many partners § Reduces provider access barriers § Important to clearly define trade channels and specify “own use provisions” to limit cross channel arbitrage Limited Distribution § Distribute through limited number of partners § May be utilized to manage drugs with somewhat restrictive REMS § Used for products with small patient population, or where special distributor capabilities are required Copyright © 2016 by the HDA Research Foundation

Certain products require special handling to help ensure product viability and regulatory compliance Special Handling: Products § Many specialty drugs have special handling requirements – temperatures may need to be held within a certain range to ensure product viability, packaging requirements Product Types § Products requiring cold chain handling may include, but are not limited to vaccines, biologics, hormones, insulin, certain antibiotics § DEA schedule II - IV drugs are highly regulated, and must be carefully monitored and managed to help prevent theft and avoid uses for which they are not intended § Distribution centers have separate areas for products requiring special handling § Cold chain products are stored in separate chilled areas within the warehouse Warehouse Handling § DEA schedule I and II controlled substances (e. g. oxycodone) are stored in locked vaults, with special procedures for access § DEA schedule III, V and IV controlled substances must be stored in caged areas built to the specifications of the DEA regulations § Cold chain products are packed in special qualified containers or boxes and may have temperature monitors or cold packs enclosed in the package In-Transit Handling § All persons and companies involved in handling DEA schedule I - IV drugs must be registered with the DEA and the inventory must be carefully tracked and reported during its supply chain journey Copyright © 2016 by the HDA Research Foundation
Different pack outs are selected for use on a seasonal basis, to account for differences in ambient temperatures Seasonal Pack out Determination Resulting Pack out Options Average Ambient Temperatures Average Temperature in August Summer Seasonal Temperature Profiles S M L Average Temperature in February Winter S M L Copyright © 2016 by the HDA Research Foundation
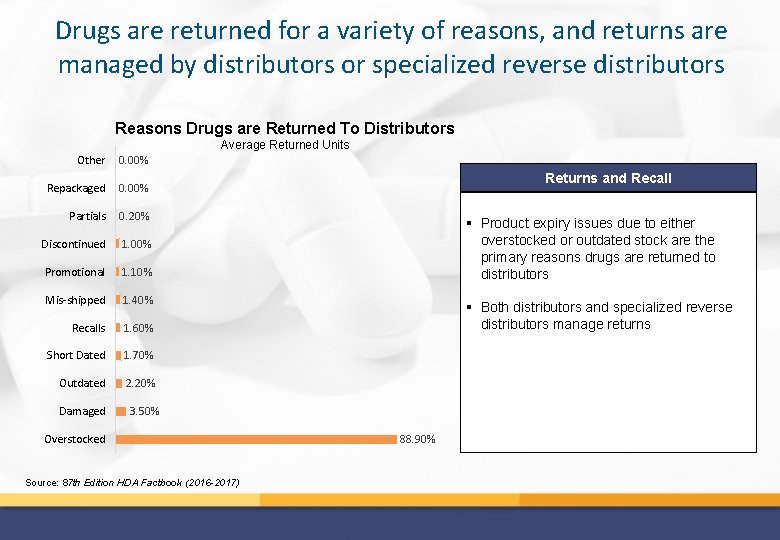
Drugs are returned for a variety of reasons, and returns are managed by distributors or specialized reverse distributors Reasons Drugs are Returned To Distributors Average Returned Units Other 0. 00% Repackaged 0. 00% Partials 0. 20% Discontinued 1. 00% Promotional 1. 10% Mis-shipped 1. 40% Recalls 1. 60% Short Dated 1. 70% Outdated 2. 20% Damaged Returns and Recall § Product expiry issues due to either overstocked or outdated stock are the primary reasons drugs are returned to distributors § Both distributors and specialized reverse distributors manage returns 3. 50% Overstocked Source: 87 th Edition HDA Factbook (2016 -2017) 88. 90%
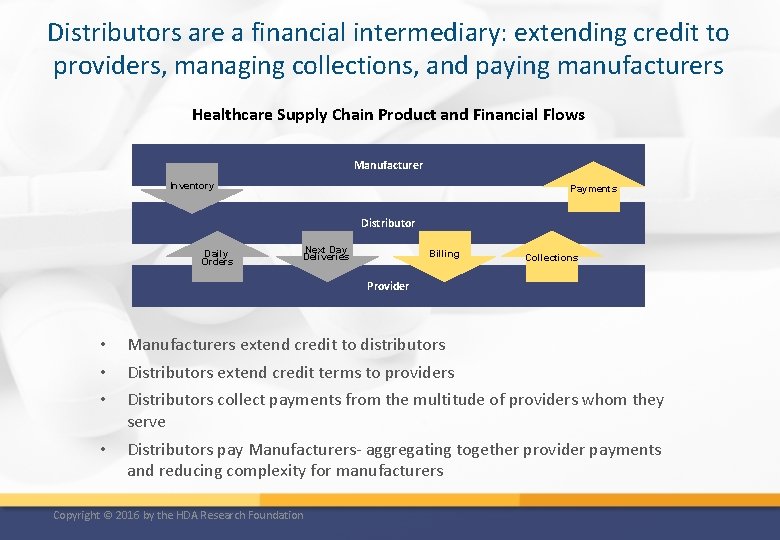
Distributors are a financial intermediary: extending credit to providers, managing collections, and paying manufacturers Healthcare Supply Chain Product and Financial Flows Manufacturer Inventory Payments Distributor Daily Orders Next Day Deliveries Billing Collections Provider • • • Manufacturers extend credit to distributors • Distributors pay Manufacturers- aggregating together provider payments and reducing complexity for manufacturers Distributors extend credit terms to providers Distributors collect payments from the multitude of providers whom they serve Copyright © 2016 by the HDA Research Foundation

Closing Thoughts • • • The U. S. pharmaceutical supply chain is highly efficient and ensures that products are safely and reliably available to a wide variety of providers, meeting patients needs Patient safety and drug efficacy must be the top priority when designing the supply chain strategy for pharmaceutical drugs – and manufacturers, distributors and providers all play important roles in delivering against this mission Strong supply chain strategies and execution support the commercial success of branded and generic manufacturers – both for existing and new product launches Manufacturers choose to create partnerships with distributors to distribute product on their behalf, and help ensure that product is available and accessible to providers Providers have many choices from whom they will procure product, and distribution partners offer critical services which support providers in delivering patient care Copyright © 2016 by the HDA Research Foundation

Glossary Copyright © 2016 by the HDA Research Foundation

Glossary of Terms These terms are used in the pharmaceutical distribution industry and may be found in the text of the 85 th Edition HDA Factbook (2014 -2015). Words or phrases defined uniquely by the cited source are listed as table notes. ANDA ASN AWP Chain Drug Stores Chargeback Cost of Goods Sold CPFR CRM DC DEA DME DTC EC EDI Employee Turnover Rate Abbreviated New Drug Application Advance Ship Notice Average Wholesale Price Drug stores with four or more stores primarily in the business of retail sales of pharmaceuticals and OTC products The amount a distributor bills back to a manufacturer when a product is sold to a customer at a contract price that is less than the distributor’s cost [Beginning inventory + purchases (net of trade discounts & returns, freight, value of inventory shrinkage and sales or use taxes) ending inventory] Collaborative Planning Forecasting and Replenishment — frequent replenishment of product based on actual and forecasted demand Customer Relationship Marketing Distribution center Drug Enforcement Administration (U. S. ) Durable Medical Equipment Direct to Consumer — pharmaceutical advertising directed to the patient, rather than to the healthcare professional Electronic Commerce Electronic Data Interchange The average number of employees divided by the number of employees leaving in the past 12 months Copyright © 2016 by the HDA Research Foundation

Glossary of Terms (cont’d) FDA FIFO Fill rate FTE’s FTL GDP GPO HBC HHC HMO HPC IMA Institutional Customers Independent Drug Stores Inside Sales Force ISD Food and Drug Administration (U. S. ) First In First Out — a method of valuing the cost of goods sold that uses the cost of the oldest items in inventory first Percent of both total sales invoice lines and total Full-Time Employees, where the proportion of full-time hours worked is reported for those who do not work full -time Full-truck load Gross Domestic Product Group Purchasing Organization — negotiates buying contracts for healthcare products at discounted fees for hospitals and health plans Health and Beauty Care – products that do not treat a therapeutic class, (e. g. , toiletries and cosmetics) Home Healthcare products Health Maintenance Organization — provides, offers or arranges coverage of health services for a fixed, prepaid premium Health and Personal Care (used interchangeably with HBC) Inventory Management Agreement Customers who purchase healthcare products without the primary intention of reselling them, including hospitals, nursing homes, prisons or institutions Drug stores with no more than one to three locations, and are not part of a larger chain Sales staff that work customer accounts from an office where calls are received for products or services Insufficient Data Copyright © 2016 by the HDA Research Foundation

Glossary of Terms (cont’d) Line Extension MAT Media Conversion Services NA NDA Non-Stock Sales Order Fill Rate OTC PBM Q/M R&D Radiographic Products Repackaging Returns RFID RONW Saleable SKU Scorecarding Dollar amount of a single invoice line (quantity multiplied by price) Moving Annual Total Fax-to-EDI or Web-based solutions to electronically capture information from non-EDI-capable trading partners Not Applicable New Drug Application Products sold by a distributor, but not put into inventory – sold mainly to chain drug warehouses The percent of both total sales invoice lines and total sales dollars ordered that are shipped without error or backorder Over-the-Counter Pharmacy Benefit Management Quarterly/Monthly Research and Development Drugs used for diagnostic tests, such as dyes, barium and drugs for nuclear diagnostics Removing product from the original package and dividing it into smaller quantity packages Products unsaleable at the retail level (damaged, outdated, overstocked, etc. ) returned to a manufacturer or distributor for credit Radiofrequency Identification Return on Net Worth Product that may be sold or re-sold and re-placed into inventory Stock Keeping Unit — code that identifies each unique product form An evaluation that assesses the order fulfillment process between trading partners Copyright © 2016 by the HDA Research Foundation

Glossary of Terms (cont’d) Specialty Distribution Specialty Pharmacy Stock Sales TRx Unit Dose Unit of Use Unsaleable VMI WAC Handles specialty products that are generally defined as two or more of the following: typically high cost ($600 or more per month); require special handling, storage and delivery; generally biologically-derived products, available in injectable, infused and occasionally oral forms; dispensed to treat individuals with chronic or rare diseases; have complex treatment regimes that require ongoing clinical monitoring and patient education; frequently have limited or exclusive product availability and distribution; used to treat therapeutic categories such as oncology, autoimmune/immune, inflammatory, etc. Typically a pharmacy function that focuses on high cost, high maintenance products that treat chronic diseases, such as rheumatology or oncology. These products are nearly always injectable, biotech products. Many are associated with complex reimbursement issues, require specialized shipping and handling, and require disease state management tools to maximize therapeutic benefit and assure patient compliance Sales of product that have been unpacked and placed in distributor inventory Total Prescription The package level for a single dose of therapy The package level for one course of therapy Healthcare products retuned to distributors or manufacturers which cannot be sold, or re-placed into inventory and re-sold Vendor Managed Inventory — replenishment is managed by the supplier based on the trading partner agreement Wholesale Acquisition Cost Copyright © 2016 by the HDA Research Foundation
- Slides: 54