Understanding Periodic Trends CHAPTER 6 Timeline 1829 J




- Slides: 18

Understanding Periodic Trends CHAPTER 6

Timeline 1829 J. W. Dobereiner l l l Classification system in which elements are grouped in triads 3 elements with similar chemical properties Didn’t work for all of the elements 1869 -Russian Chemist, Dmitri Mendeleev: l l l Designed a Periodic Table 1869 Elements are listed in order of increasing atomic mass 1870—German Lother Meyer published his table describing periodic trends

Timeline continued 1913 -British Scientist, Henry Moseley: Designed the Modern Periodic Table l Elements are listed in order of increasing atomic # l Based on Periodic Law: physical & chemical properties of elements are periodic functions of their atomic #s l

PERIODICITY There are general trends in the properties of atoms and their ions. These trends can be explained using the periodic table and the electron configurations of the atoms.


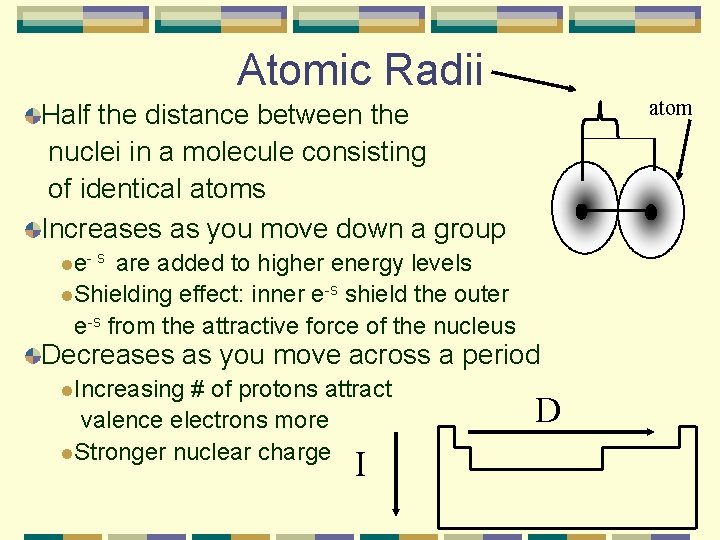
Atomic Radii atom Half the distance between the nuclei in a molecule consisting of identical atoms Increases as you move down a group l e- s are added to higher energy levels l. Shielding effect: inner e-s shield the outer e-s from the attractive force of the nucleus Decreases as you move across a period l. Increasing # of protons attract valence electrons more l. Stronger nuclear charge I D

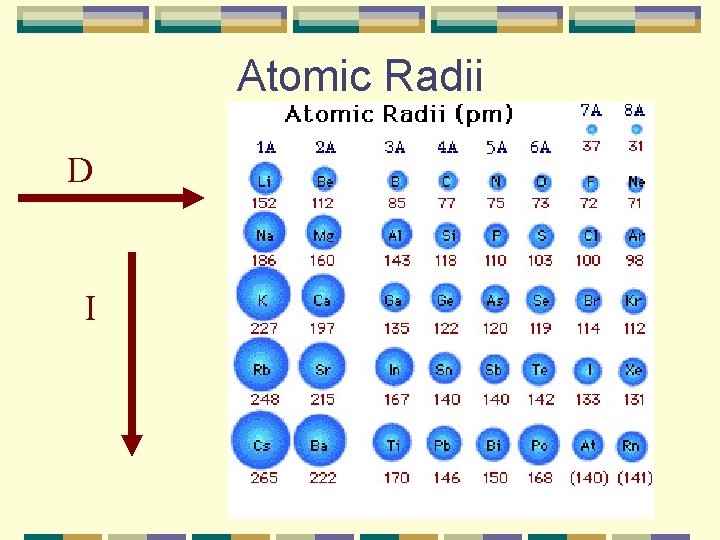
Atomic Radii D I

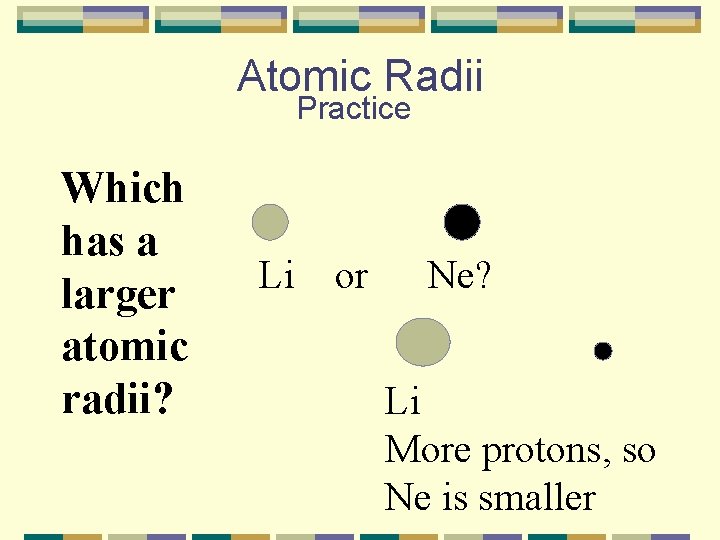
Atomic Radii Practice Which has a larger atomic radii? Li or Ne? Li More protons, so Ne is smaller

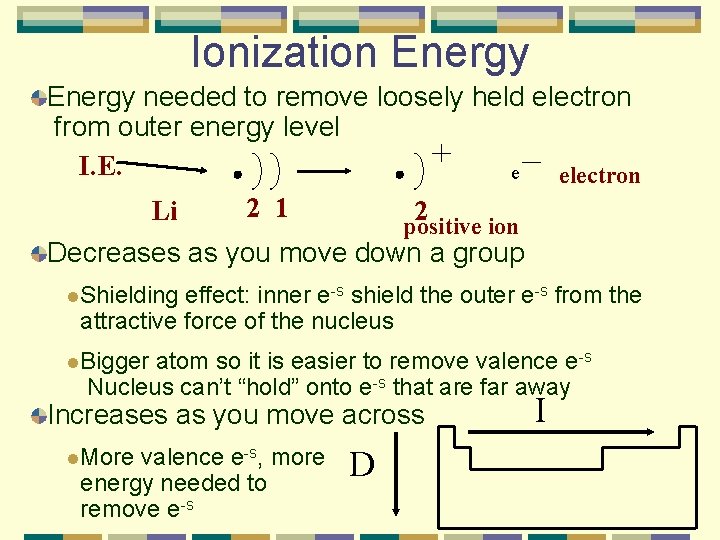
Ionization Energy needed to remove loosely held electron from outer energy level I. E. e electron Li 2 1 2 positive ion Decreases as you move down a group l. Shielding effect: inner e-s shield the outer e-s from the attractive force of the nucleus l. Bigger atom so it is easier to remove valence e-s Nucleus can’t “hold” onto e-s that are far away Increases as you move across l. More valence e-s, more energy needed to remove e-s D I

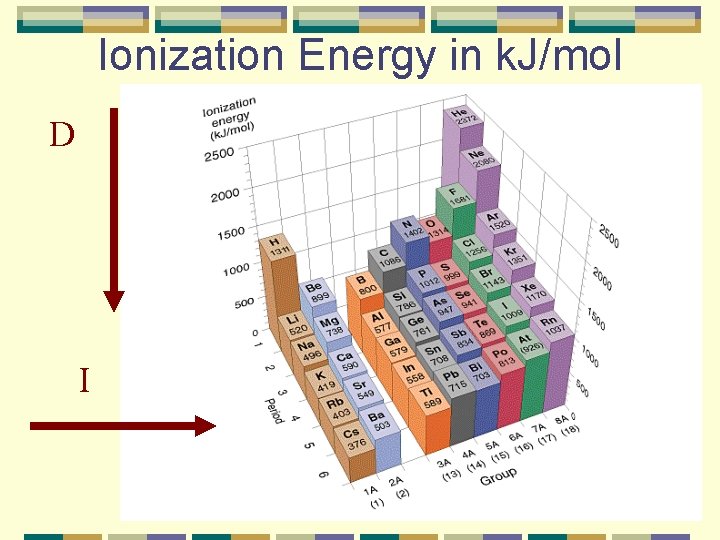
Ionization Energy in k. J/mol D I

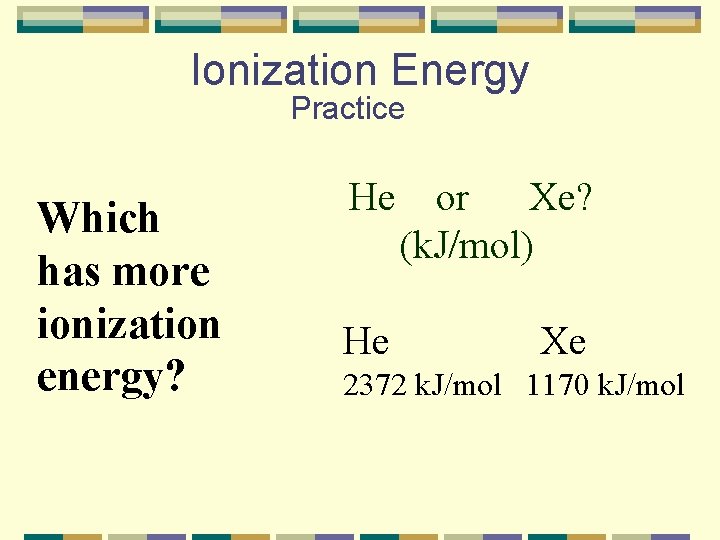
Ionization Energy Practice Which has more ionization energy? He or Xe? (k. J/mol) He Xe 2372 k. J/mol 1170 k. J/mol

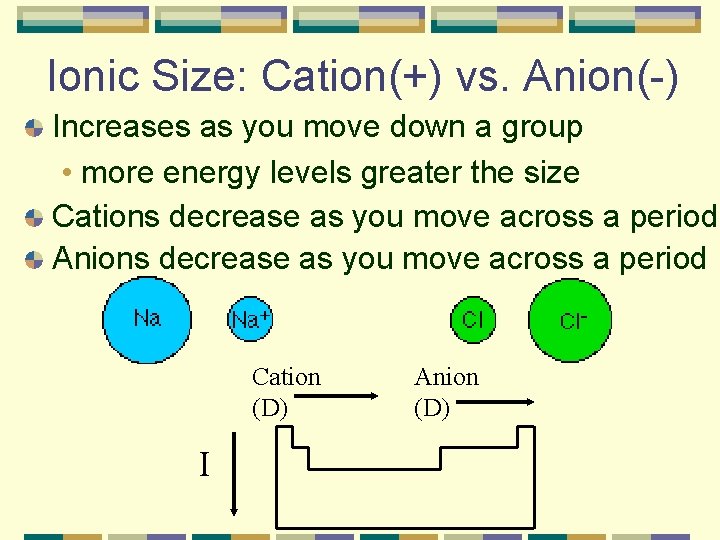
Ionic Size: Cation(+) vs. Anion(-) Increases as you move down a group • more energy levels greater the size Cations decrease as you move across a period Anions decrease as you move across a period Cation (D) I Anion (D)

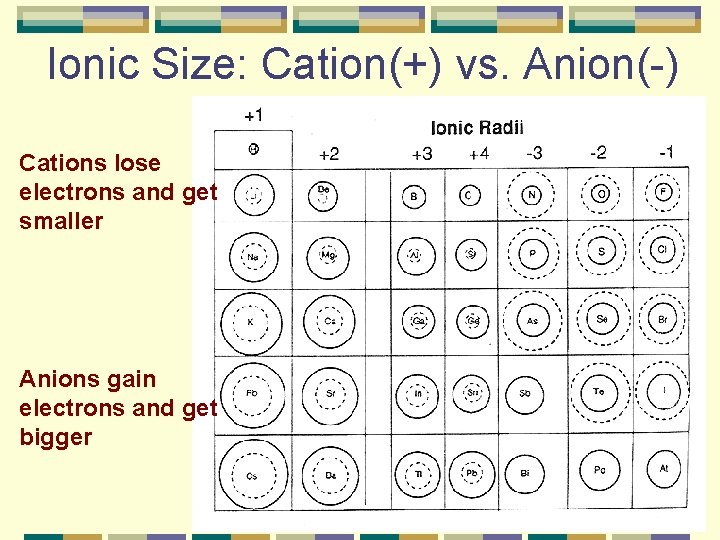
Ionic Size: Cation(+) vs. Anion(-) Cations lose electrons and get smaller Anions gain electrons and get bigger

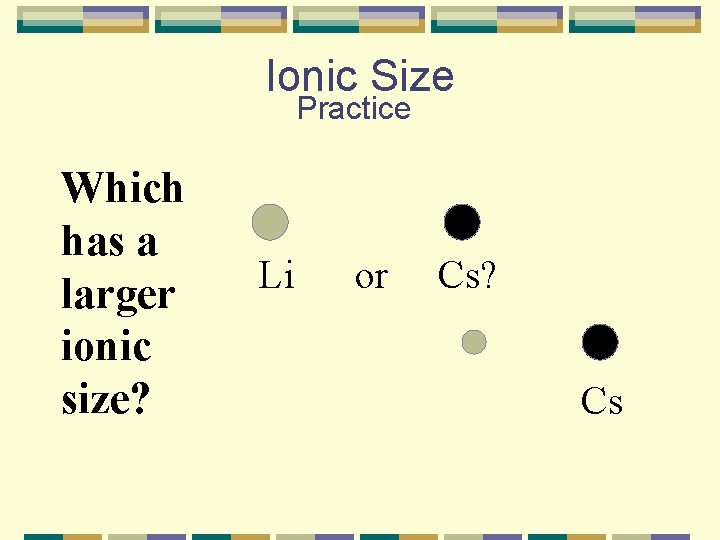
Ionic Size Practice Which has a larger ionic size? Li or Cs? Cs

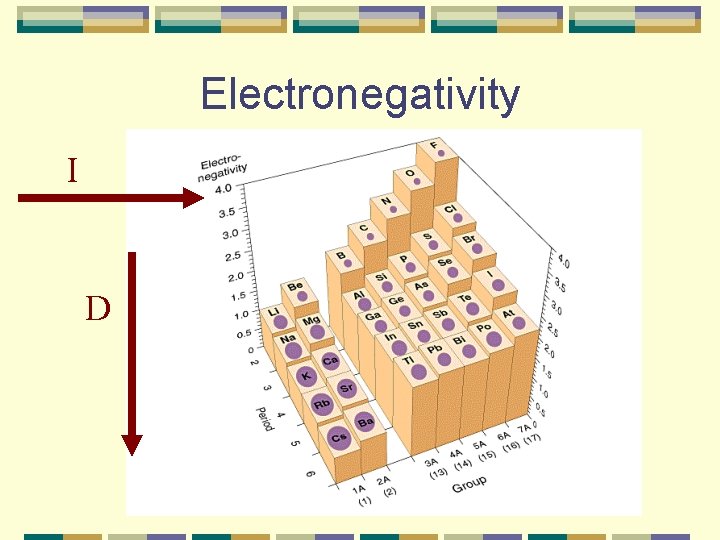
Electronegativity The ability of an atom to attract e-s to itself when it is chemically combined with another element. Decreases as you move down a group l Bigger the atom, the harder it is for the nucleus to attract e-s Increases as you move across a period l More valence e-s, easier to gain e-s than lose (MAGIC 8) l Noble gases are omitted because they don’t form I many compounds D

Electronegativity I D

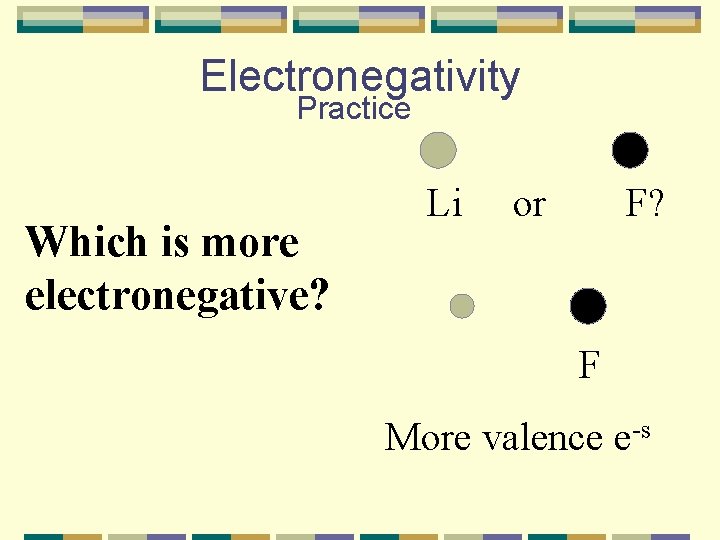
Electronegativity Practice Which is more electronegative? Li or F? F More valence e-s

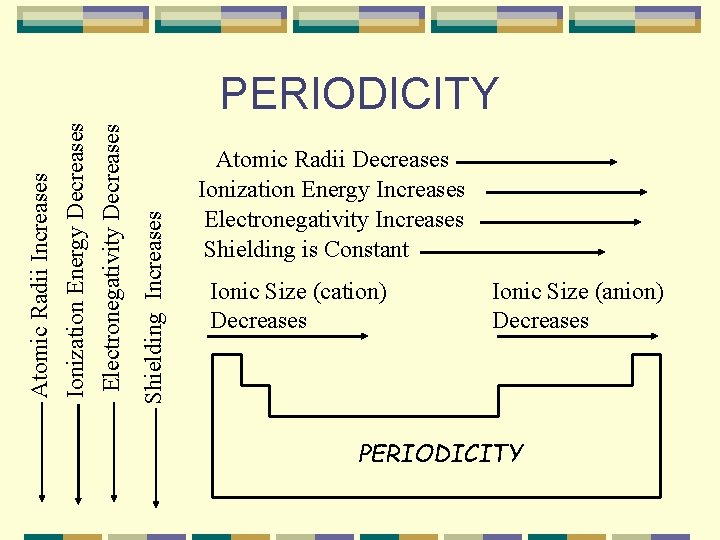
Shielding Increases Atomic Radii Increases Ionization Energy Decreases Electronegativity Decreases PERIODICITY Atomic Radii Decreases Ionization Energy Increases Electronegativity Increases Shielding is Constant Ionic Size (cation) Decreases Ionic Size (anion) Decreases PERIODICITY