Understanding FDA Regulatory Process and Requirements for Medical




















- Slides: 43

Understanding FDA Regulatory Process and Requirements for Medical Device Classification Moj Eram, Ph. D Senior Consultant ARC Experts, LLC 6 April, 2016

Overview § § § FDA Overview – Regulatory agency Device Classifications Device Clearance/Approval Requirements Quality System Regulation (QSR) Fees and statistics Summary

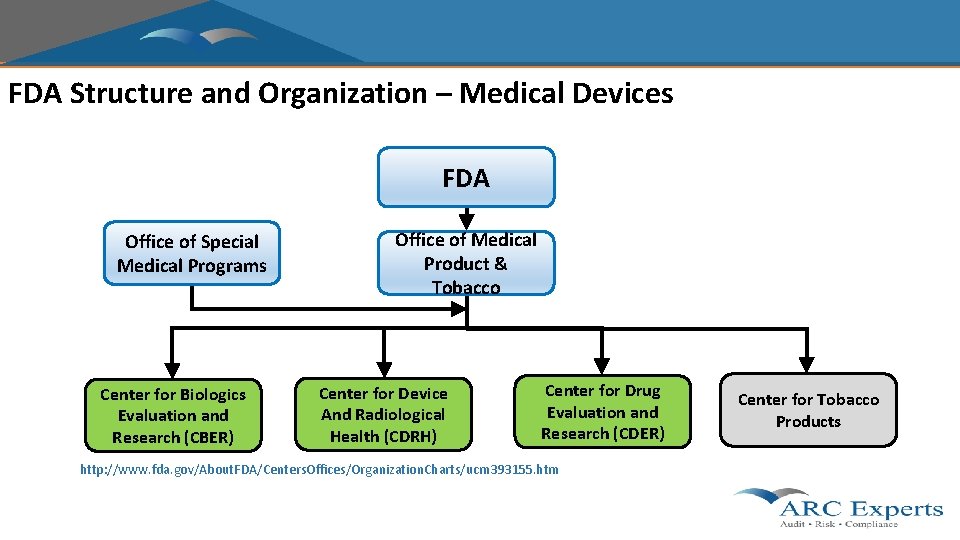
FDA Structure and Organization – Medical Devices FDA Office of Special Medical Programs Center for Biologics Evaluation and Research (CBER) Office of Medical Product & Tobacco Center for Device And Radiological Health (CDRH) Center for Drug Evaluation and Research (CDER) http: //www. fda. gov/About. FDA/Centers. Offices/Organization. Charts/ucm 393155. htm Center for Tobacco Products

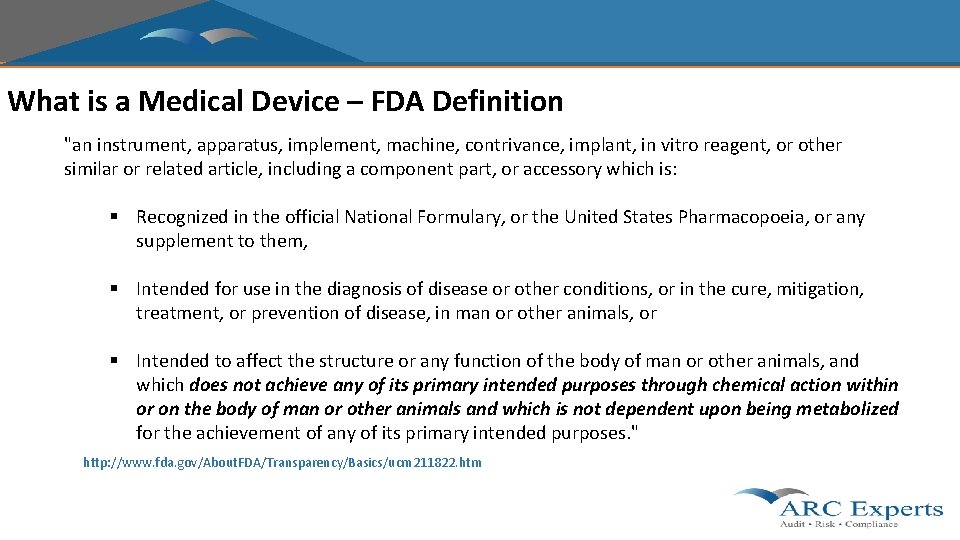
What is a Medical Device – FDA Definition "an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part, or accessory which is: § Recognized in the official National Formulary, or the United States Pharmacopoeia, or any supplement to them, § Intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals, or § Intended to affect the structure or any function of the body of man or other animals, and which does not achieve any of its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes. " http: //www. fda. gov/About. FDA/Transparency/Basics/ucm 211822. htm

Medical Devices Classification Establishment § FDA is the sole federal agency regulating safety and effectiveness of medical devices. § Most medial devices can be classified using the description of device in Title 21 of the Code of Federal Regulations (CFR), Parts 862 -892. § Approximately 1700 different generic types of devices. § Grouped into 16 medical specialties (i. e. anesthesiology, cardiovascular, neurology, …) – referred to as panels. § These panels make recommendation for device classification. § Each generic types is assigned to one of the three regulatory classes. § Classification is based on risk and level of control necessary to ensure the safety and effectiveness of device.

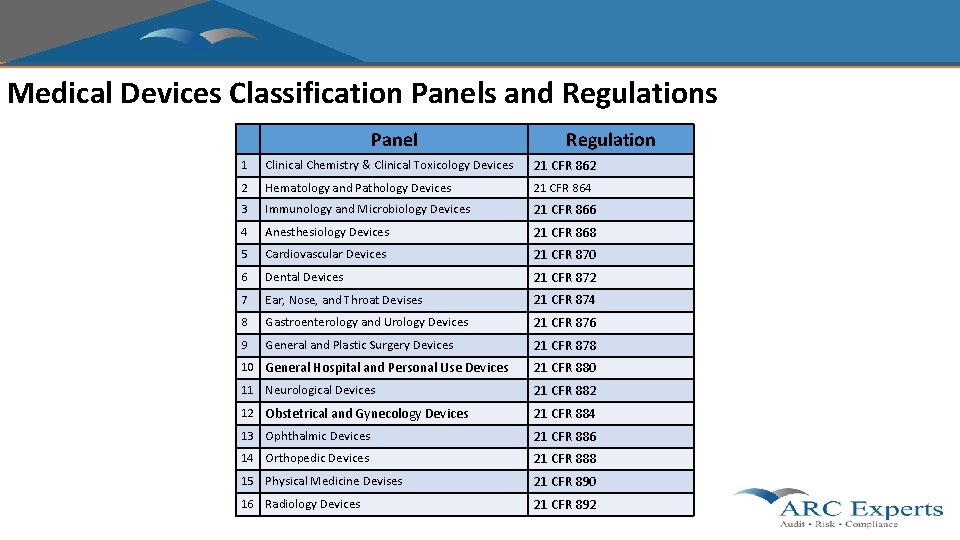
Medical Devices Classification Panels and Regulations Panel Regulation 1 Clinical Chemistry & Clinical Toxicology Devices 21 CFR 862 2 Hematology and Pathology Devices 21 CFR 864 3 Immunology and Microbiology Devices 21 CFR 866 4 Anesthesiology Devices 21 CFR 868 5 Cardiovascular Devices 21 CFR 870 6 Dental Devices 21 CFR 872 7 Ear, Nose, and Throat Devises 21 CFR 874 8 Gastroenterology and Urology Devices 21 CFR 876 9 General and Plastic Surgery Devices 21 CFR 878 10 General Hospital and Personal Use Devices 21 CFR 880 11 Neurological Devices 21 CFR 882 12 Obstetrical and Gynecology Devices 21 CFR 884 13 Ophthalmic Devices 21 CFR 886 14 Orthopedic Devices 21 CFR 888 15 Physical Medicine Devises 21 CFR 890 16 Radiology Devices 21 CFR 892

Risk-Based Classification - Factors may Affect Risk Design § Devices design should incorporate the principle of inherent Safety. Manufacturer § Manufacturing processes must well planned and under control. § Validated processes to ensure conforming output and enhance safety. Intended Use § Objective intent of the manufacturer on how the device will be used. § The indented use will define the Scope of use, and in particular, places where the device is not intended for use.

Risk-Based Classification - Factors may Affect Risk User Experience, Education, and Training § Define the anticipated user. § Identify the expected use’s skill level (home use, hospital use, complex devices). Device Should not Compromise § The clinical condition and safety of patients. § Consider safety or health of users. Inherent Risk/Benefit Analysis § The benefit outweighs the risk.

What Does it Mean to Classify a Medical Device § Determining the classification is the first step in obtaining FDA clearance/approval for a medical device. § FDA defines the classes on a risk-based approval process into three classes: § Class I – FDA Product Registration § Class II – Pre-Market Notification – 510(k) § Class III – Pre-Market Approval (PMA) § Product development under general and special control based on the class. § Product approval requires a compliant Quality Management System (QMS).

Medical Devices Classification – Class I § Class I devices are deemed to be low to moderate risk and are therefore subject to the least regulatory controls (i. e. dental floss, bandages, tongue depressor). § 47% of medical devices; 95% exempt form regulatory approval process. § No FDA pre-approval is needed and most are exempt form 510(k) premarket notification. § Must register the device and company with FDA and pay fee. § Under general control. § Quality System Regulations (QSR) - mainly for production control and post market surveillance, record keeping and reporting.

Medical Devices Classification – Class II § Class II devices are moderate to high risk and require greater regulatory controls to provide reasonable assurance of the device’s safety and effectiveness (i. e. Blood pressure kit, contact lenses, needles). § 43% of medial devices. § Require Pre-Market Notification (PMN) or 510(k). § Under general and special control. § Performance data – Bench testing, animal (if needed), clinical (if needed). § Some Class II devices may be exempt from 510(k) clearance. § Formal risk assessment.

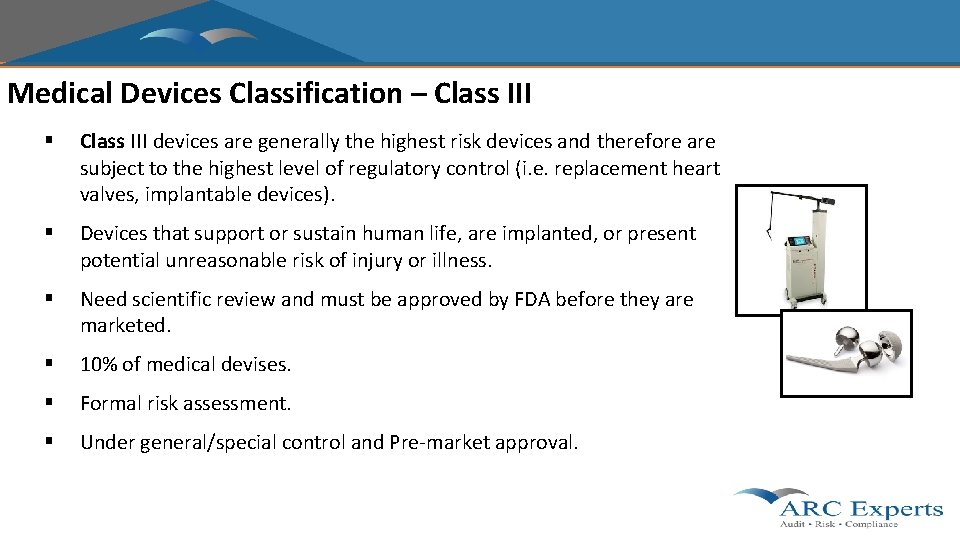
Medical Devices Classification – Class III § Class III devices are generally the highest risk devices and therefore are subject to the highest level of regulatory control (i. e. replacement heart valves, implantable devices). § Devices that support or sustain human life, are implanted, or present potential unreasonable risk of injury or illness. § Need scientific review and must be approved by FDA before they are marketed. § 10% of medical devises. § Formal risk assessment. § Under general/special control and Pre-market approval.

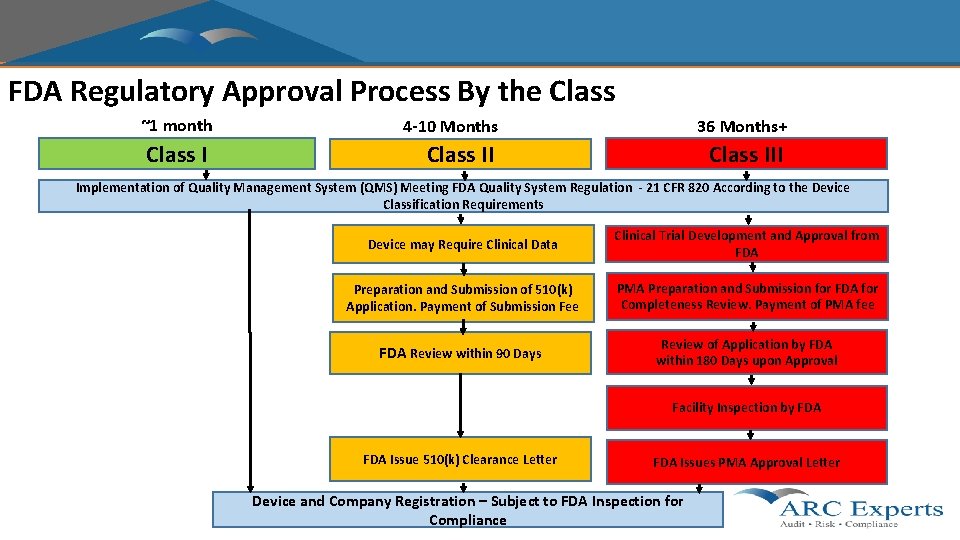
FDA Regulatory Approval Process By the Class ~1 month 4 -10 Months 36 Months+ Class III Implementation of Quality Management System (QMS) Meeting FDA Quality System Regulation - 21 CFR 820 According to the Device Classification Requirements Device may Require Clinical Data Clinical Trial Development and Approval from FDA Preparation and Submission of 510(k) Application. Payment of Submission Fee PMA Preparation and Submission for FDA for Completeness Review. Payment of PMA fee FDA Review within 90 Days Review of Application by FDA within 180 Days upon Approval Facility Inspection by FDA Issue 510(k) Clearance Letter FDA Issues PMA Approval Letter Device and Company Registration – Subject to FDA Inspection for Compliance

How to Determine Device Classification § http: //www. fda. gov/Medical. Devices/default. htm § Use basic description of the device to perform search – in “Classification Database”. § If the device panel is known for the device, go to the panel, identify the device and corresponding regulation. § Look for a device similar to your device already in the market. § Read product description most similar to your device. § Look for device name, regulation number, submission type, device class, and product code.

How to Determine Device Classification - continued § If the device is classified as Class I or II and if It is not exempt, a 510(k) clearance will be required for marketing. § All devices classified as exempt are subject to the limitation on exemption. § Limitation of device exemptions are covered under 21 CFR 862 -892.

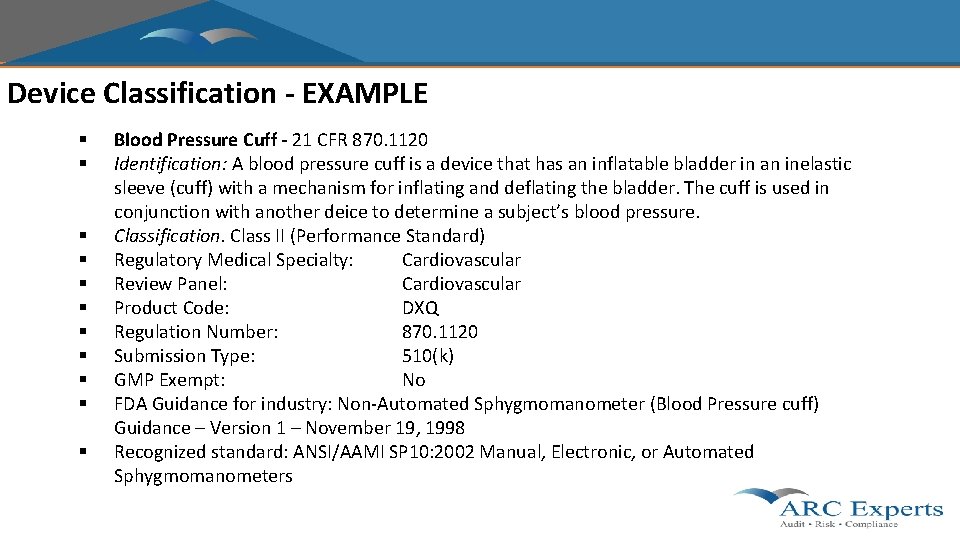
Device Classification - EXAMPLE § § § Blood Pressure Cuff - 21 CFR 870. 1120 Identification: A blood pressure cuff is a device that has an inflatable bladder in an inelastic sleeve (cuff) with a mechanism for inflating and deflating the bladder. The cuff is used in conjunction with another deice to determine a subject’s blood pressure. Classification. Class II (Performance Standard) Regulatory Medical Specialty: Cardiovascular Review Panel: Cardiovascular Product Code: DXQ Regulation Number: 870. 1120 Submission Type: 510(k) GMP Exempt: No FDA Guidance for industry: Non-Automated Sphygmomanometer (Blood Pressure cuff) Guidance – Version 1 – November 19, 1998 Recognized standard: ANSI/AAMI SP 10: 2002 Manual, Electronic, or Automated Sphygmomanometers

Pre-Market Notification – 510(k) § For manufacturers seeking new device approval in the US and for FDA to classify that device. § Using substantial equivalent threshold – predicate device specification, you can demonstrate that your device is as safe and effective as a product(s) legally marketed in the US. § The 510(k) Program: Evaluating Substantial Equivalence in Premarket Notification [510(k)] http: //www. fda. gov/downloads/Medical. Devices/Device. Regulationand. Guidanc e/Guidance. Documents/UCM 284443. pdf § QSR, Labeling, Comparative Information (Clinical Data), Indication for Use, etc.

510(k) Process – Substantial Equivalence (SE) § A 510(k) submission requires demonstration of Substantial Equivalence (SE) to another legally US marketed device. Substantial Equivalence means that the new device is at least as safe and effective as the predicate. § A device is Substantially Equivalent if, in comparison to a predicate it: § Has the same intended use as the predicate; and § Has the same technological characteristics as the predicate, Or § Has the same intended use as the predicate, and § Has different technological characteristics and the information submitted to FDA § Does not raise new or different question of safety and effectiveness; and § Demonstrates that the device is at least as safe and effective as the legally marketed device.

510(k) Process – Substantial Equivalence (SE) § A claim of SE does not mean the new and predicate devices must be identical. § SE is established with respect to: § Intended use, § Design, § Energy used or delivered, § Materials, § Chemical composition, § Manufacturing process, § Performance, safety, § Effectiveness, labeling, § Biocompatibility § Standards, and § Other characteristics, as applicable.

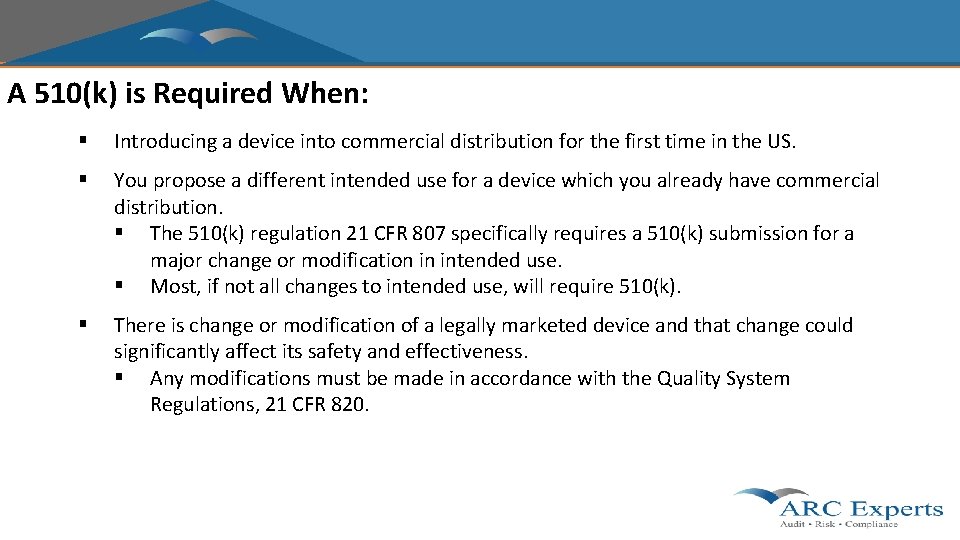
A 510(k) is Required When: § Introducing a device into commercial distribution for the first time in the US. § You propose a different intended use for a device which you already have commercial distribution. § The 510(k) regulation 21 CFR 807 specifically requires a 510(k) submission for a major change or modification in intended use. § Most, if not all changes to intended use, will require 510(k). § There is change or modification of a legally marketed device and that change could significantly affect its safety and effectiveness. § Any modifications must be made in accordance with the Quality System Regulations, 21 CFR 820.

510(k) Submission Requirements § § § Description of the new device Labeling Identification of predicate Device(s) Narrative and tabular comparison Predicate device’s intended use and indication(s) for Use Technological characteristic of the device Principles of operation Software documentation (If applicable) Sterility Information (If applicable) Statement of declaration of conformance to applicable standards and guidance documents Summaries of any performance testing Administrative Requirements § Truthfulness and accuracy statement § 510(k) summary § Payment of a user fee

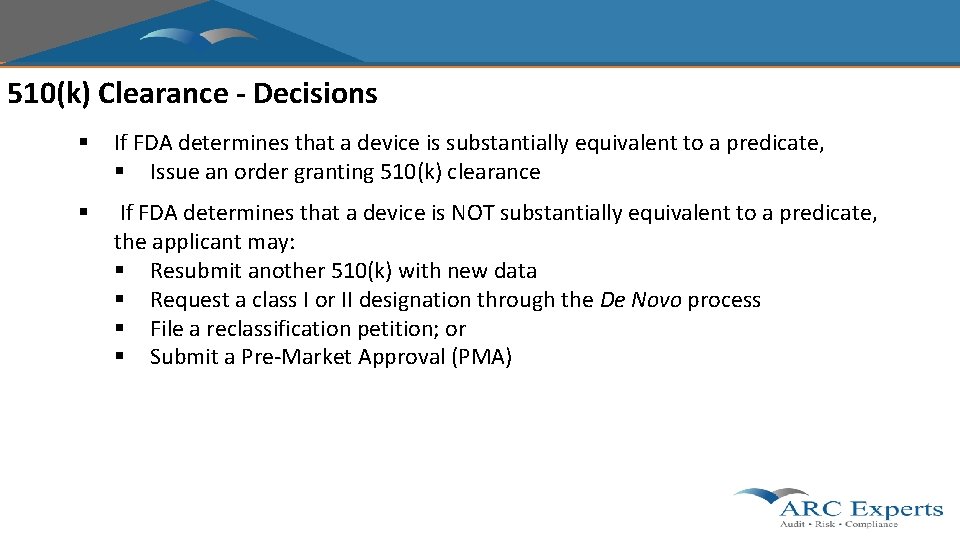
510(k) Clearance - Decisions § If FDA determines that a device is substantially equivalent to a predicate, § Issue an order granting 510(k) clearance § If FDA determines that a device is NOT substantially equivalent to a predicate, the applicant may: § Resubmit another 510(k) with new data § Request a class I or II designation through the De Novo process § File a reclassification petition; or § Submit a Pre-Market Approval (PMA)

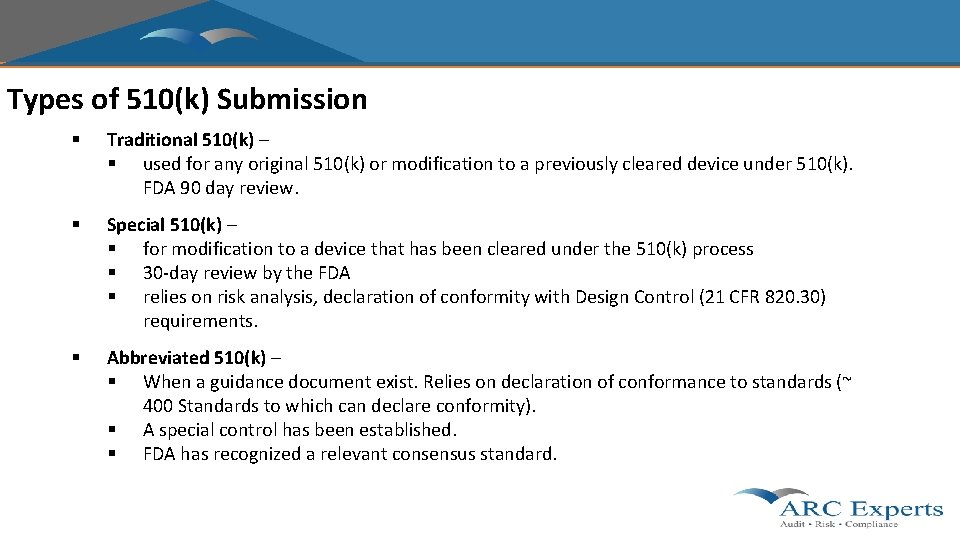
Types of 510(k) Submission § Traditional 510(k) – § used for any original 510(k) or modification to a previously cleared device under 510(k). FDA 90 day review. § Special 510(k) – § for modification to a device that has been cleared under the 510(k) process § 30 -day review by the FDA § relies on risk analysis, declaration of conformity with Design Control (21 CFR 820. 30) requirements. § Abbreviated 510(k) – § When a guidance document exist. Relies on declaration of conformance to standards (~ 400 Standards to which can declare conformity). § A special control has been established. § FDA has recognized a relevant consensus standard.

Who is Required to submit a 510(k) § Domestic US manufacturers introducing a device to the US market. § Finished device manufacturer § Accessories to finished device § Contract manufacturers are NOT required to submit 510(k) § Specification developers introducing device to the US market § Develop specification for a finished device, but the device is manufactured under contract by another entity § Repackers or relabelers who make significant labeling changes or whose operations significantly affect the device. § Foreign manufacturers/exporters or US representative of foreign manufacturers/exporters introducing a device to the US market.

De Novo Classification § FDA will consider De Novos for devices that are not within a device type that has been classified by FD&CA and classified by written notice as “novel”. (EXAMPLE: Esophageal cooling device) § To qualify, the device must be both: § Novel § Low to moderate risk § Two options for De Novo classification: § If receive NSE determination by the FDA in response to a 510(k) submission, within 30 days submit a De Novo Request to make a risk-based evaluation for classification of Class I or II. § If determined there are no legally marketed device (no predicate), may submit a De Novo request risk-based classification of the device into class I or II without submitting 510(k). § Description of the device. § Justification for recommendation for De Novo classification. § Information/data to support the recommendation – Bench, animal, human clinical data.

De Novo Review Process § FDA has 120 days to review the request. § If FDA classifies the device into class I or II § Special Control guidance issue § Device can be used as predicate for future 510(k) submissions § If FDA determines that the device remains Class III § PMA approval is required for market

Pre-Market Approval (PMA) – Class III § No substantial equivalence (SE) found. Must demonstrate safety and effectiveness of a new device by valid scientific evidence. § Convenes an advisory committee, providing nonbinding recommendation to FDA. § Inspection of manufacturer’s facility to QSR. § Safe and effectiveness demonstration § Non-clinical and clinical trial data, device description, intended use claims, manufacturing data, labeling § Investigational Device Exemption (IDE) § Pre-clinical studies, clinical trial protocol approval

Pre-Market Approval (PMA) Submission Requirements § § § Complete description of the device and components (Drawings, photo, etc. ) Detailed description of the methods, facilities, and controls used in manufacturing Prepared labeling, advertising literature, training materials, etc. Software documentation (if applicable) Sterility Information (if applicable) Biocompatibility information Extensive clinical trials Animal studies Bench testing Published and unpublished literatures Bibliography of all published reports concerning device safety and efficacy.

Humanitarian Device Exemption (HDE) § Humanitarian Use Device (HUD) are devices for treating or diagnosing a disease or condition that affect fewer than 4000 in the US annually. § Must submit HDE application to FDA. § Similar to PMA requirements, except for effectiveness requirements of PMA. § Application does not require to contain the result of scientifically valid clinical investigations to demonstrate device effectiveness. § Must submit enough information to determine that the device does not pose significant risk of illness or injury. § Only be used after IRB approval for FDA approved indication. § labeling - must state that the device is a HUD, and although it is authorized by Federal Law, the effectiveness of the device for the indication has not been demonstrated.

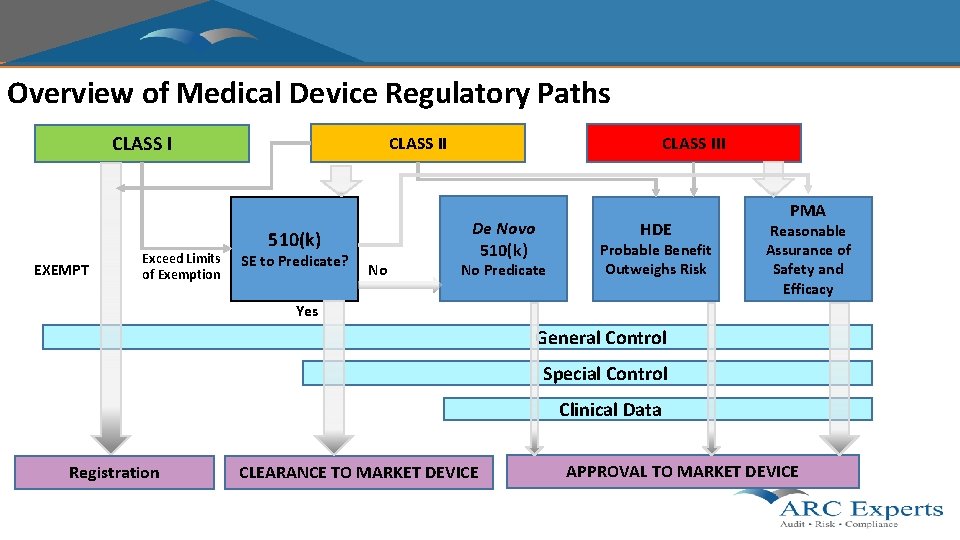
Overview of Medical Device Regulatory Paths CLASS I EXEMPT Exceed Limits of Exemption CLASS II 510(k) SE to Predicate? No CLASS III De Novo 510(k) HDE No Predicate Probable Benefit Outweighs Risk PMA Reasonable Assurance of Safety and Efficacy Yes General Control Special Control Clinical Data Registration CLEARANCE TO MARKET DEVICE APPROVAL TO MARKET DEVICE

Medical Devices – General Control § General controls are regulatory requirements by FDA, and apply to all medical devices, unless exempt by regulations. § General Control, described in FD&CA Sections 501, 502, 510, 516, 518, 519, & 520, include provisions of the activities pertaining to: § Adulteration § Misbranding § Device Registration and listing § Premarket Notification (510(k)) § Banned Devises § Notification, repair, replacement, refund, reimbursement, recall § Records and report § Restricted device § General Provisions including Good Manufacturing Practice (GMP)

Medical Devices – Special Control § Special Controls are regulatory requirements for Class II devices, in addition to general controls. § Special controls are device specific and include: § Performance standards – Design Control § Patient registries § Special labeling requirements § Pre-market data requirements § Post-market surveillance § Guidelines/standards

Quality System § A medical device quality system is designed to assure that products are safe and effective for their intended use, and § consistently meet the specifications defined by results of clinical and/or detailed technical design and validation.

Quality System Regulation (QSR) – 21 CFR 820 § Medical device manufacturer must establish and follow a quality systems to help ensure that their products consistently meet applicable requirements and specifications. § QSR also known as current Good Manufacturing Practices (c. GMP). § Requirements are not prescriptive, but provide framework of basic requirements for manufacturers to follow. § Place focus on pre and post marketing activities. § Manufacturer must develop a Quality Management System (QMS) commensurate with: § Risk presented by the device § Complexity of device and manufacturing processes § Size and complexity of organization

Quality System Regulation (QSR) Elements – 21 CFR 820 § § § § Management Controls Design Controls Materials Controls Document Controls Purchasing Controls Production and Process Controls Facilities and Equipment Controls Document, Record and Change Controls § § § Corrective and Preventive Action Labeling Controls Handling, Storage and Distribution Controls Servicing Controls Statistical Techniques

Quality System Regulation (QSR) – Design Control – 21 CFR 820. 30 § § § § Establishing intended use and design input Design Plan Deign Review – periodic phase gate reviews of design & development process Design Verification – Confirmation that Design output conforms to design input Design Validation – Conformance to the user need and intended use Design Transfer – Translation of design into manufacturing specifications Clear and complete documentation of design control process – Design History File (DHF)

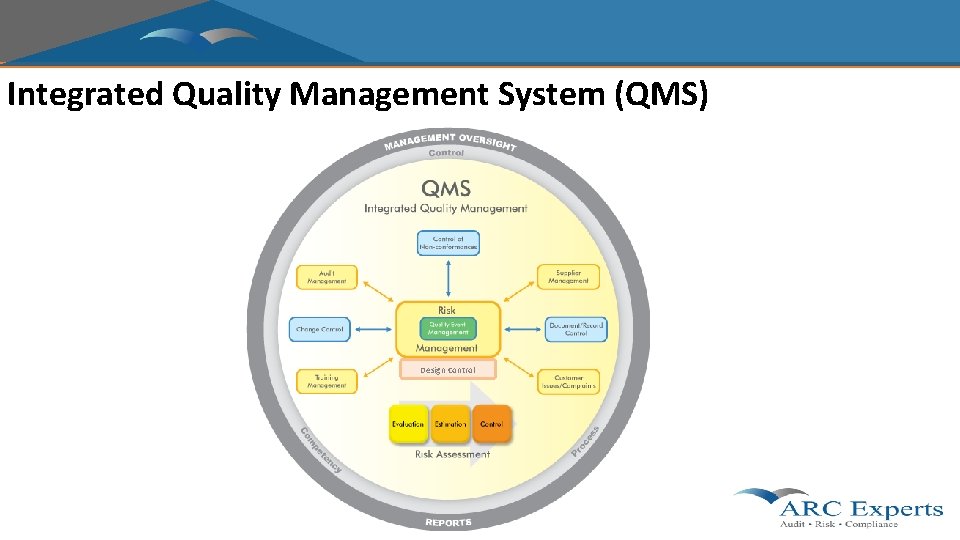
Integrated Quality Management System (QMS) Design Control

Resources § Code of Federal Regulations (CFR) – 21 Parts 800 to 1299 – Key provisions: § Part 801 - Labeling § Part 803 – Medical Device reporting (MDR) § Part 807 – Registration, Listing and 510(k) § Part 812 – IDE § Part 814 – PMA § Part 820 – QSR § Part 822 – Post market surveillance § Part 860 – Classification § FDA, CDRH: http: //www. fda. gov/Training/CDRHLearn/

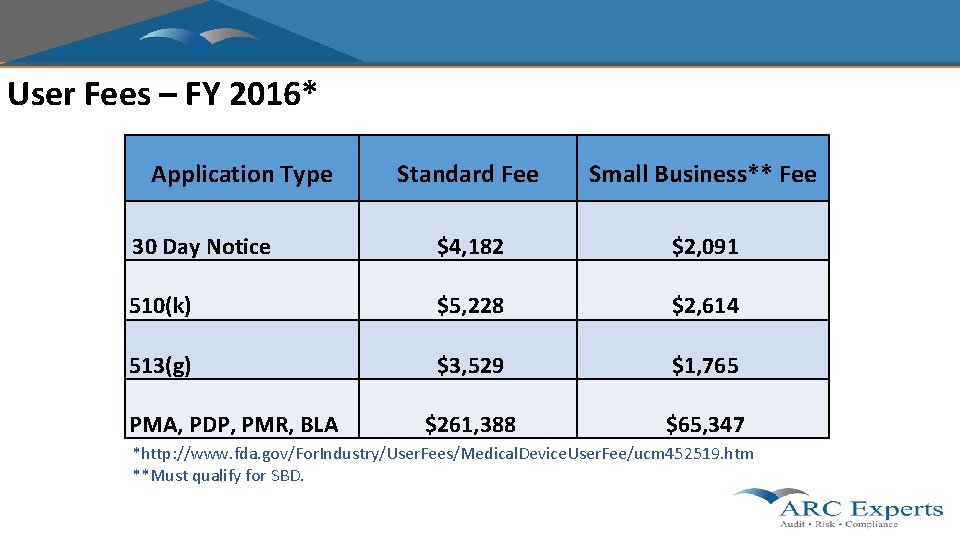
User Fees – FY 2016* Application Type Standard Fee Small Business** Fee 30 Day Notice $4, 182 $2, 091 510(k) $5, 228 $2, 614 513(g) $3, 529 $1, 765 $261, 388 $65, 347 PMA, PDP, PMR, BLA *http: //www. fda. gov/For. Industry/User. Fees/Medical. Device. User. Fee/ucm 452519. htm **Must qualify for SBD.

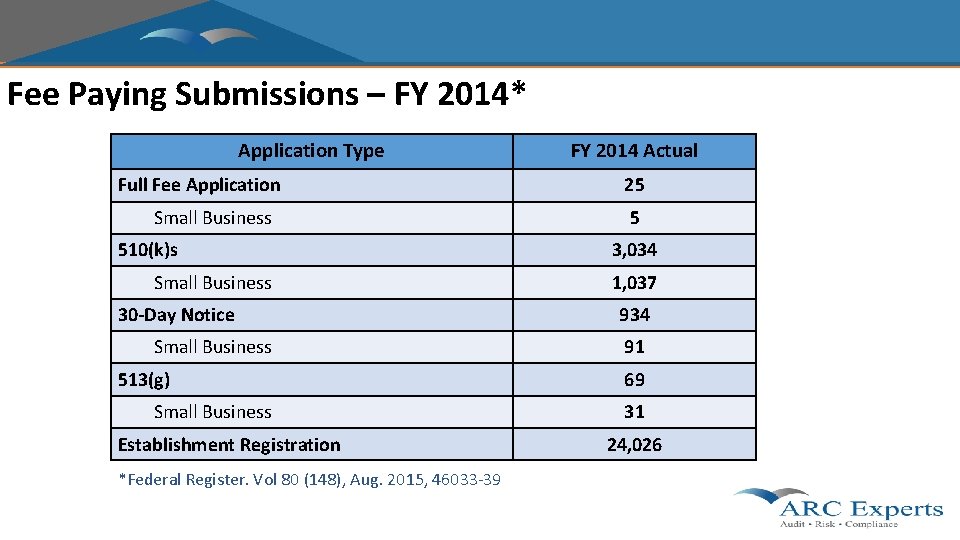
Fee Paying Submissions – FY 2014* Application Type Full Fee Application Small Business 510(k)s Small Business 30 -Day Notice Small Business 513(g) Small Business Establishment Registration *Federal Register. Vol 80 (148), Aug. 2015, 46033 -39 FY 2014 Actual 25 5 3, 034 1, 037 934 91 69 31 24, 026

Summary Start the process of device classification early. Determine the regulatory path. Determine quality and control requirements for your device. Establish communication with FDA to improve the quality of your submission and likelihood of approval. § Develop your product according to the QSR, design control requirements (Class II & III). § §

Thank you Moj Eram, Ph. D Senior Consultant ARC Experts, LLC moj. eram@arcexperts. com 801 -230 -8611

 Regulatory change management process
Regulatory change management process Hepburn osteometric board
Hepburn osteometric board Fda vs brown and williamson
Fda vs brown and williamson Chapter 22 regulatory and advisory agencies
Chapter 22 regulatory and advisory agencies Chapter 2 pharmacy law ethics and regulatory agencies
Chapter 2 pharmacy law ethics and regulatory agencies Diversity and regulatory challenges
Diversity and regulatory challenges Warehousing development and regulatory authority
Warehousing development and regulatory authority Oig pharma compliance guidance
Oig pharma compliance guidance Michigan licensing and regulatory affairs
Michigan licensing and regulatory affairs Legal regulatory and political issues
Legal regulatory and political issues Pharmaceutical regulatory and compliance congress
Pharmaceutical regulatory and compliance congress Legal and regulatory framework of microfinance in india
Legal and regulatory framework of microfinance in india Pharmaceutical regulatory and compliance congress
Pharmaceutical regulatory and compliance congress Pharmaceutical regulatory and compliance congress
Pharmaceutical regulatory and compliance congress Fspos
Fspos Typiska novell drag
Typiska novell drag Nationell inriktning för artificiell intelligens
Nationell inriktning för artificiell intelligens Returpilarna
Returpilarna Varför kallas perioden 1918-1939 för mellankrigstiden
Varför kallas perioden 1918-1939 för mellankrigstiden En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Särskild löneskatt för pensionskostnader
Särskild löneskatt för pensionskostnader Tidböcker
Tidböcker A gastrica
A gastrica Densitet vatten
Densitet vatten Datorkunskap för nybörjare
Datorkunskap för nybörjare Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Hur skriver man en tes
Hur skriver man en tes Autokratiskt ledarskap
Autokratiskt ledarskap Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Påbyggnader för flakfordon
Påbyggnader för flakfordon Vätsketryck formel
Vätsketryck formel Svenskt ramverk för digital samverkan
Svenskt ramverk för digital samverkan Jag har gått inunder stjärnor text
Jag har gått inunder stjärnor text Presentera för publik crossboss
Presentera för publik crossboss Vad är ett minoritetsspråk
Vad är ett minoritetsspråk Bat mitza
Bat mitza Treserva lathund
Treserva lathund Mjälthilus
Mjälthilus Claes martinsson
Claes martinsson Cks
Cks Byggprocessen steg för steg
Byggprocessen steg för steg Mat för idrottare
Mat för idrottare Verktyg för automatisering av utbetalningar
Verktyg för automatisering av utbetalningar Rutin för avvikelsehantering
Rutin för avvikelsehantering