Understanding evaporation Aseel Samaro Introduction Have you ever















- Slides: 19

Understanding evaporation Aseel Samaro



Introduction Have you ever thought about what happens to the water particles when a puddle is left in the sunshine, or to the water produced when you sweat? The answer is the water has evaporated. What does this actually mean and how is it different from boiling?

Examples of evaporation § If you spill some water on a surface and leave it in the sunshine, after a few hours the water particles will no longer be present and there is no sign of the spill. § The water has evaporated. § The water particles have gained sufficient energy from their surroundings to become a gas.

§ When clothes are hung out to dry, the air particles in the wind transfer energy by movement (kinetic energy) to the water particles in the clothes. § These gain enough energy to change from a liquid into a vapour.

§ Smells from perfumes are also an example of evaporation. § The perfume particles gain enough energy by heat from the body, or by kinetic energy from air particles, to become a vapour.

Describe one other example of evaporation. Where does the energy needed for evaporation to take place come from?

Differences between boiling and evaporation § Evaporation occurs at any temperature between the melting point of a liquid and its boiling point. § It happens only at the surface of the liquid. § Some of the surface particles gain enough energy from the surroundings from heat, or from kinetic energy, to leave the surrounding particles in the liquid and become a vapour. § Over time, all the particles at the surface will evaporate.

Is evaporation more likely to take place at temperatures near the boiling point or near the melting point? Explain your answer. it will take place near the boiling point the temperature is higher, so there is more heat transferred to the liquid particles; so more will evaporate What are the main differences between boiling and evaporation? Boiling is where the whole of the liquid changes to a gas at a fixed temperature. Evaporation is where some of the particles escape to become a gas; at temperatures between the melting point and the boiling point


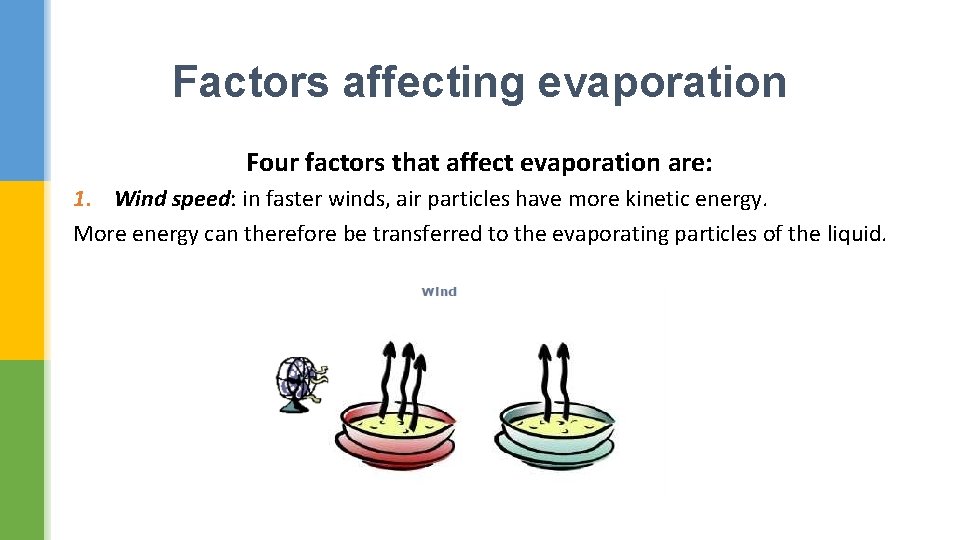
Factors affecting evaporation Four factors that affect evaporation are: 1. Wind speed: in faster winds, air particles have more kinetic energy. More energy can therefore be transferred to the evaporating particles of the liquid.

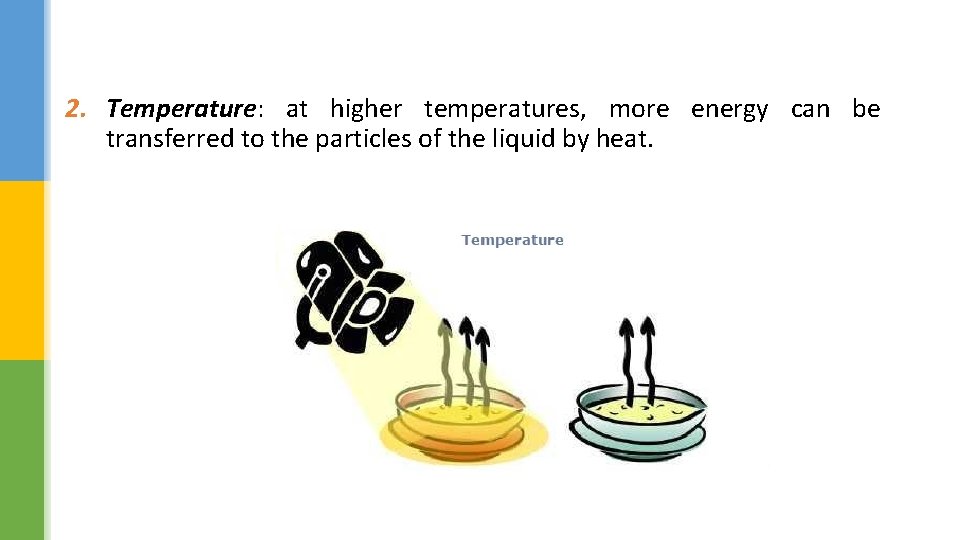
2. Temperature: at higher temperatures, more energy can be transferred to the particles of the liquid by heat.


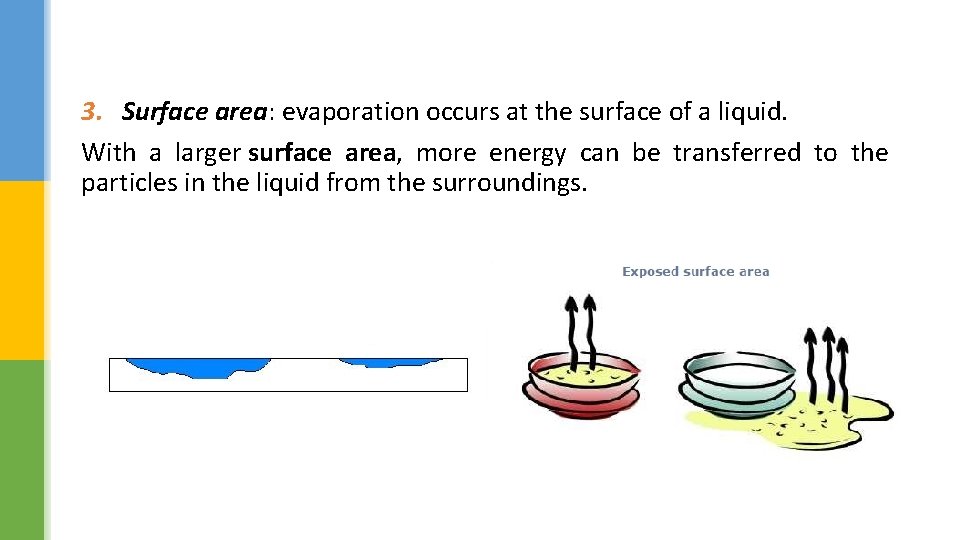
3. Surface area: evaporation occurs at the surface of a liquid. With a larger surface area, more energy can be transferred to the particles in the liquid from the surroundings.

4. Strength of the intermolecular forces: some liquids, such as alcohol, have weaker intermolecular forces than others, such as water. Therefore, with the same amount of energy transferred from the surroundings, more alcohol particles than water particles will escape as a gas.

Explain the factors that affect the rate of evaporation in this process. The more heat transferred by the Sun the higher the rate of evaporation. The larger the surface area the higher the rate of evaporation. The stronger the surface wind the higher the rate of evaporation. Which of the factors above are likely to have the biggest effect on the rate of evaporation? Explain your answer. The effect of temperature; the energy transferred by heat will directly affect the kinetic energy of more surface particles; changing the state from liquid to gas more quickly. salt production

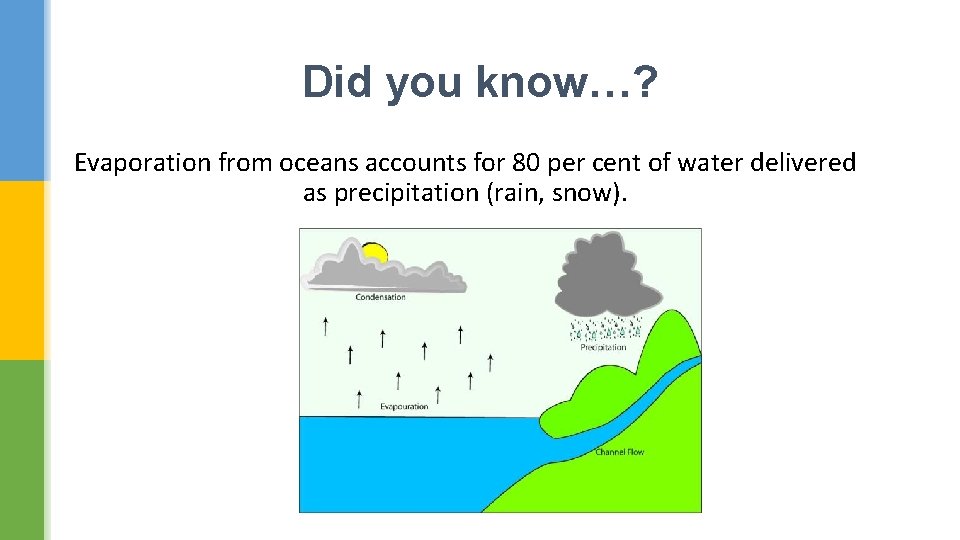
Did you know…? Evaporation from oceans accounts for 80 per cent of water delivered as precipitation (rain, snow).

Thank you
 How to identify pure aseel
How to identify pure aseel Aseel yousef
Aseel yousef Lab samaro
Lab samaro Lab-samaro
Lab-samaro Lab samaro
Lab samaro Samaro property
Samaro property Ever ancient ever new
Ever ancient ever new Clarified it
Clarified it Have you ever looked
Have you ever looked Ever tried ever failed no matter
Ever tried ever failed no matter Have you ever done anything dangerous
Have you ever done anything dangerous Have you ever wondered
Have you ever wondered Did you know
Did you know Have you ever read the book
Have you ever read the book Folkways
Folkways Have you ever climbed a mountain
Have you ever climbed a mountain Have you ever been to the water spout
Have you ever been to the water spout Have you ever ridden a camel?
Have you ever ridden a camel? Have you ever climbed a mountain?
Have you ever climbed a mountain? Have you ever seen a greenhouse
Have you ever seen a greenhouse