Understanding Electron Configurations Using Quantum Numbers Moving from

Understanding Electron Configurations Using Quantum Numbers
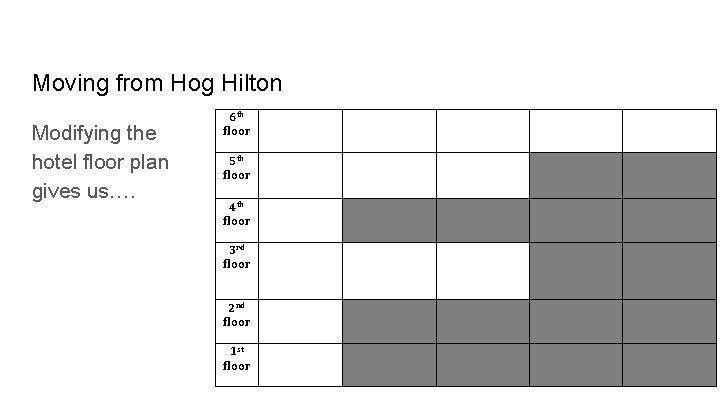
Moving from Hog Hilton Modifying the hotel floor plan gives us…. 6 th floor 5 th floor 4 th floor 3 rd floor 2 nd floor 1 st floor
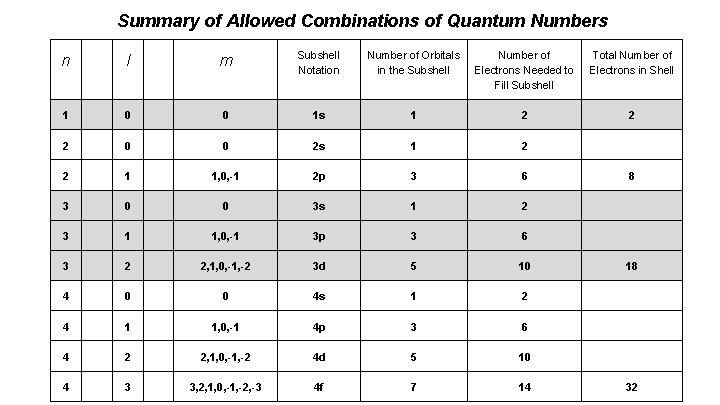
Summary of Allowed Combinations of Quantum Numbers n l m Subshell Notation Number of Orbitals in the Subshell Number of Electrons Needed to Fill Subshell Total Number of Electrons in Shell 1 0 0 1 s 1 2 2 2 0 0 2 s 1 2 2 1 1, 0, -1 2 p 3 6 3 0 0 3 s 1 2 3 1 1, 0, -1 3 p 3 6 3 2 2, 1, 0, -1, -2 3 d 5 10 4 0 0 4 s 1 2 4 1 1, 0, -1 4 p 3 6 4 2 2, 1, 0, -1, -2 4 d 5 10 4 3 3, 2, 1, 0, -1, -2, -3 4 f 7 14 8 18 32
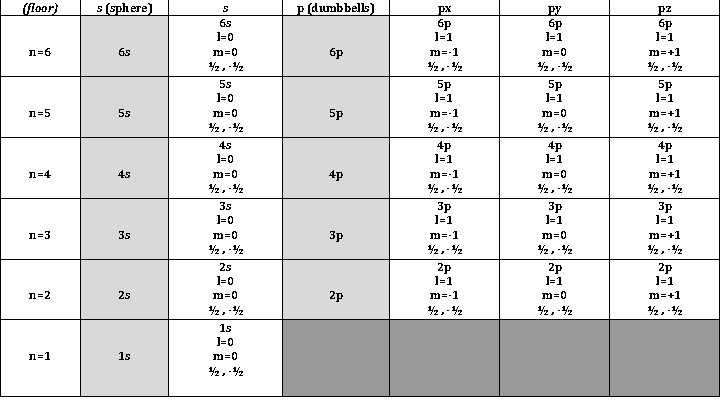
(floor) s (sphere) n=6 6 s n=5 5 s n=4 4 s n=3 3 s n=2 2 s n=1 1 s s 6 s l=0 m=0 ½ , -½ 5 s l=0 m=0 ½ , -½ 4 s l=0 m=0 ½ , -½ 3 s l=0 m=0 ½ , -½ 2 s l=0 m=0 ½ , -½ 1 s l=0 m=0 ½ , -½ p (dumbbells) 6 p 5 p 4 p 3 p 2 p px 6 p l=1 m=-1 ½ , -½ 5 p l=1 m=-1 ½ , -½ 4 p l=1 m=-1 ½ , -½ 3 p l=1 m=-1 ½ , -½ 2 p l=1 m=-1 ½ , -½ py 6 p l=1 m=0 ½ , -½ 5 p l=1 m=0 ½ , -½ 4 p l=1 m=0 ½ , -½ 3 p l=1 m=0 ½ , -½ 2 p l=1 m=0 ½ , -½ pz 6 p l=1 m=+1 ½ , -½ 5 p l=1 m=+1 ½ , -½ 4 p l=1 m=+1 ½ , -½ 3 p l=1 m=+1 ½ , -½ 2 p l=1 m=+1 ½ , -½
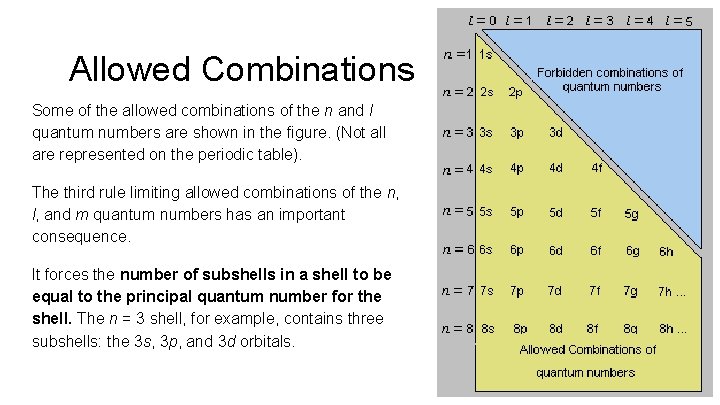
Allowed Combinations Some of the allowed combinations of the n and l quantum numbers are shown in the figure. (Not all are represented on the periodic table). The third rule limiting allowed combinations of the n, l, and m quantum numbers has an important consequence. It forces the number of subshells in a shell to be equal to the principal quantum number for the shell. The n = 3 shell, for example, contains three subshells: the 3 s, 3 p, and 3 d orbitals.
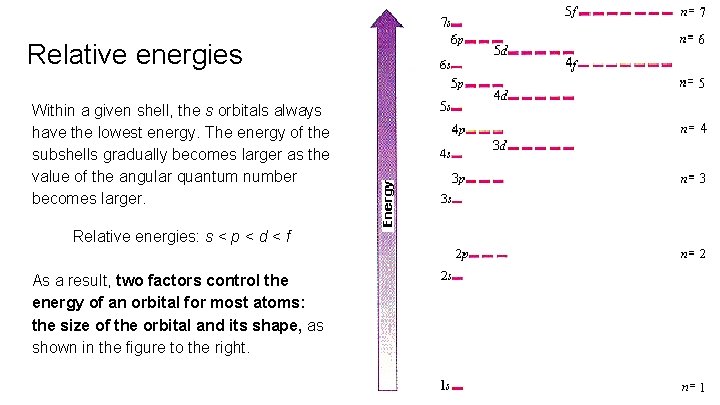
Relative energies Within a given shell, the s orbitals always have the lowest energy. The energy of the subshells gradually becomes larger as the value of the angular quantum number becomes larger. Relative energies: s < p < d < f As a result, two factors control the energy of an orbital for most atoms: the size of the orbital and its shape, as shown in the figure to the right.
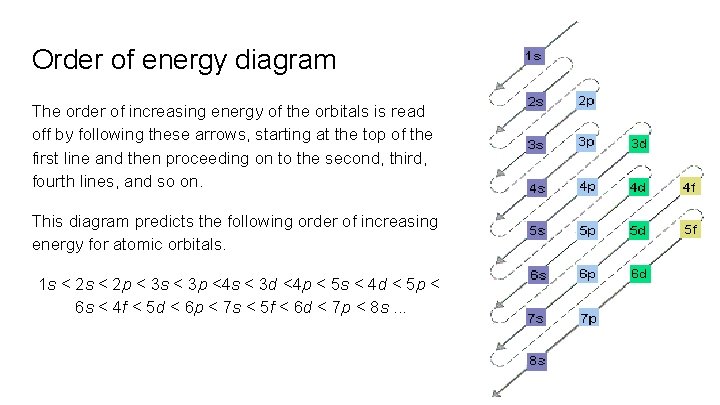
Order of energy diagram The order of increasing energy of the orbitals is read off by following these arrows, starting at the top of the first line and then proceeding on to the second, third, fourth lines, and so on. This diagram predicts the following order of increasing energy for atomic orbitals. 1 s < 2 p < 3 s < 3 p <4 s < 3 d <4 p < 5 s < 4 d < 5 p < 6 s < 4 f < 5 d < 6 p < 7 s < 5 f < 6 d < 7 p < 8 s. . .
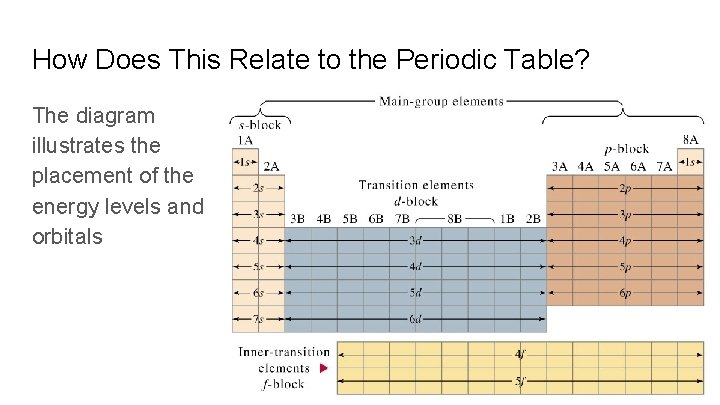
How Does This Relate to the Periodic Table? The diagram illustrates the placement of the energy levels and orbitals
- Slides: 8