Underreporting of Patient Reported Outcomes PRO in Myeloproliferative
- Slides: 1

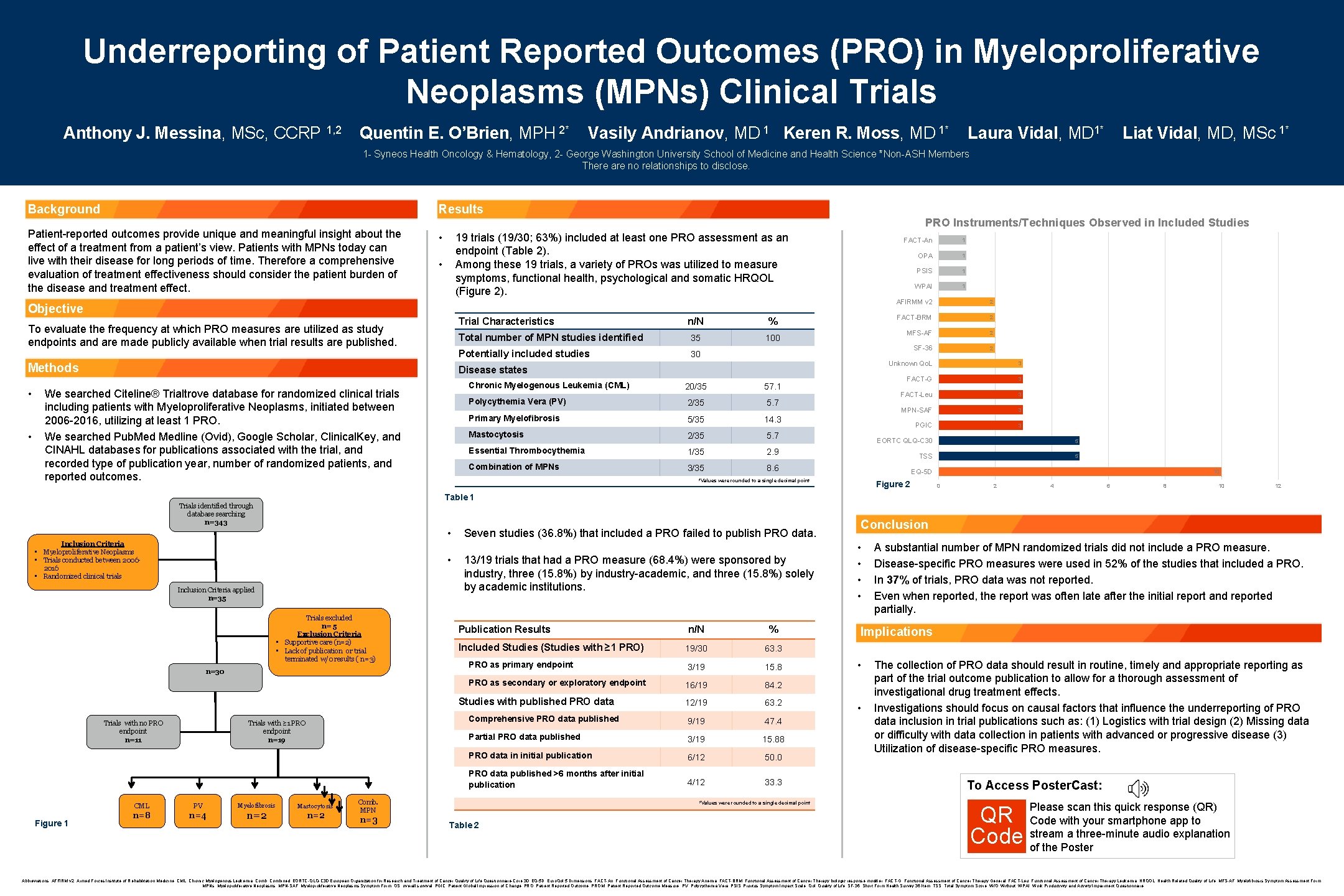
Underreporting of Patient Reported Outcomes (PRO) in Myeloproliferative Neoplasms (MPNs) Clinical Trials Anthony J. Messina, MSc, CCRP 1, 2 Quentin E. O’Brien, MPH 2* Vasily Andrianov, MD 1 Keren R. Moss, MD 1* Laura Vidal, MD 1* Liat Vidal, MD, MSc 1* 1 - Syneos Health Oncology & Hematology, 2 - George Washington University School of Medicine and Health Science *Non-ASH Members There are no relationships to disclose. Background Results Patient-reported outcomes provide unique and meaningful insight about the effect of a treatment from a patient’s view. Patients with MPNs today can live with their disease for long periods of time. Therefore a comprehensive evaluation of treatment effectiveness should consider the patient burden of the disease and treatment effect. PRO Instruments/Techniques Observed in Included Studies • 19 trials (19/30; 63%) included at least one PRO assessment as an endpoint (Table 2). Among these 19 trials, a variety of PROs was utilized to measure symptoms, functional health, psychological and somatic HRQOL (Figure 2). • Objective Trial Characteristics To evaluate the frequency at which PRO measures are utilized as study endpoints and are made publicly available when trial results are published. Methods • • n/N % Total number of MPN studies identified 35 100 Potentially included studies 30 Disease states Chronic Myelogenous Leukemia (CML) We searched Citeline® Trialtrove database for randomized clinical trials including patients with Myeloproliferative Neoplasms, initiated between 2006 -2016, utilizing at least 1 PRO. We searched Pub. Medline (Ovid), Google Scholar, Clinical. Key, and CINAHL databases for publications associated with the trial, and recorded type of publication year, number of randomized patients, and reported outcomes. 20/35 57. 1 2/35 5. 7 Primary Myelofibrosis 5/35 14. 3 Mastocytosis 2/35 5. 7 Essential Thrombocythemia 1/35 2. 9 Combination of MPNs 3/35 8. 6 Polycythemia Vera (PV) FACT-An 1 OPA 1 PSIS 1 WPAI 1 AFIRMM v 2 2 FACT-BRM 2 MFS-AF 2 SF-36 2 Unknown Qo. L 3 FACT-G 3 FACT-Leu 3 MPN-SAF 3 PGIC 3 EORTC QLQ-C 30 5 TSS 5 EQ-5 D *Values were rounded to a single decimal point Figure 2 10 0 2 4 6 8 10 12 Table 1 Trials identified through database searching n=343 • Inclusion Criteria • Myeloproliferative Neoplasms • Trials conducted between 20062016 • Randomized clinical trials • Inclusion Criteria applied n=35 Trials excluded n= 5 Exclusion Criteria • Supportive care (n=2) • Lack of publication or trial terminated w/0 results ( n=3) n=30 Seven studies (36. 8%) that included a PRO failed to publish PRO data. 13/19 trials that had a PRO measure (68. 4%) were sponsored by industry, three (15. 8%) by industry-academic, and three (15. 8%) solely by academic institutions. Publication Results n/N % 19/30 63. 3 PRO as primary endpoint 3/19 15. 8 PRO as secondary or exploratory endpoint 16/19 84. 2 12/19 63. 2 Comprehensive PRO data published 9/19 47. 4 Partial PRO data published 3/19 15. 88 PRO data in initial publication 6/12 50. 0 PRO data published >6 months after initial publication 4/12 33. 3 Included Studies (Studies with ≥ 1 PRO) Studies with published PRO data Trials with no PRO endpoint n=11 Figure 1 Trials with ≥ 1 PRO endpoint n=19 CML PV n=8 n=4 Myelofibrosis n=2 Mastocytosis n=2 Comb. MPN n=3 *Values were rounded to a single decimal point Table 2 Conclusion • • A substantial number of MPN randomized trials did not include a PRO measure. Disease-specific PRO measures were used in 52% of the studies that included a PRO. In 37% of trials, PRO data was not reported. Even when reported, the report was often late after the initial report and reported partially. Implications • • The collection of PRO data should result in routine, timely and appropriate reporting as part of the trial outcome publication to allow for a thorough assessment of investigational drug treatment effects. Investigations should focus on causal factors that influence the underreporting of PRO data inclusion in trial publications such as: (1) Logistics with trial design (2) Missing data or difficulty with data collection in patients with advanced or progressive disease (3) Utilization of disease-specific PRO measures. To Access Poster. Cast: QR Code Please scan this quick response (QR) Code with your smartphone app to stream a three-minute audio explanation of the Poster © 2019 All rights reserved | Confidential | For Syneos Health™ use only Abbreviations: AFFIRM v 2, Armed Forces Institute of Rehabilitation Medicine; CML, Chronic Myelogenous Leukemia; Comb, Combined; EORTC-QLQ-C 30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30; EQ-5 D, Euro. Qol 5 Dimensions; FACT-An, Functional Assessment of Cancer Therapy Anemia; FACT-BRM, Functional Assessment of Cancer Therapy biologic response modifier; FACT-G, Functional Assessment of Cancer Therapy General; FACT-Leu, Functional Assessment of Cancer Therapy Leukemia; HRQOL, Health Related Quality of Life; MFS-AF, Myelofibrosis Symptom Assessment Form; MPNs, Myeloproliferative Neoplasms; MPN-SAF, Myeloproliferative Neoplasms Symptom Form; OS, overall survival; PGIC, Patient Global Impression of Change; PRO, Patient Reported Outcome; PROM, Patient Reported Outcome Measure; PV, Polycythemia Vera; PSIS, Pruritus Symptom Impact Scale; Qol, Quality of Life; SF-36, Short Form Health Survey 36 Item; TSS, Total Symptom Score; W/O, Without; WPAI, Work Productivity and Activity Impairment Questionnaire