Uncertainty in Data Accuracy How close a measurement

















- Slides: 35

Uncertainty in Data

Accuracy • How close a measurement comes to an accepted value Test worth 100 pts. You scored 98

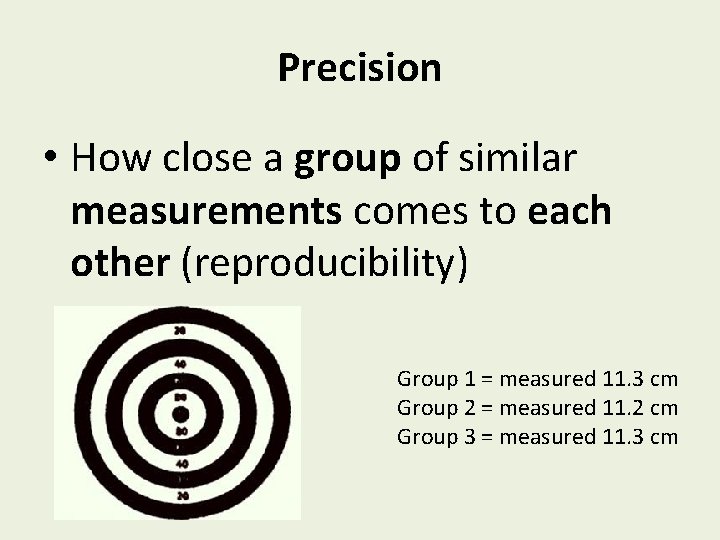
Precision • How close a group of similar measurements comes to each other (reproducibility) Group 1 = measured 11. 3 cm Group 2 = measured 11. 2 cm Group 3 = measured 11. 3 cm

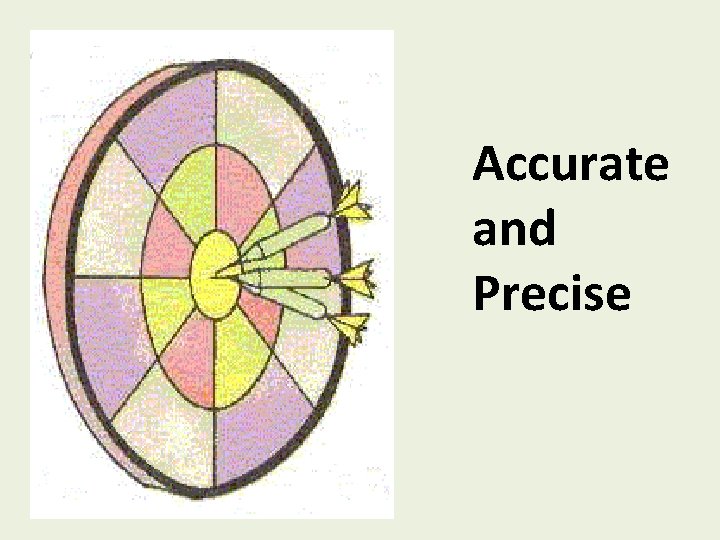
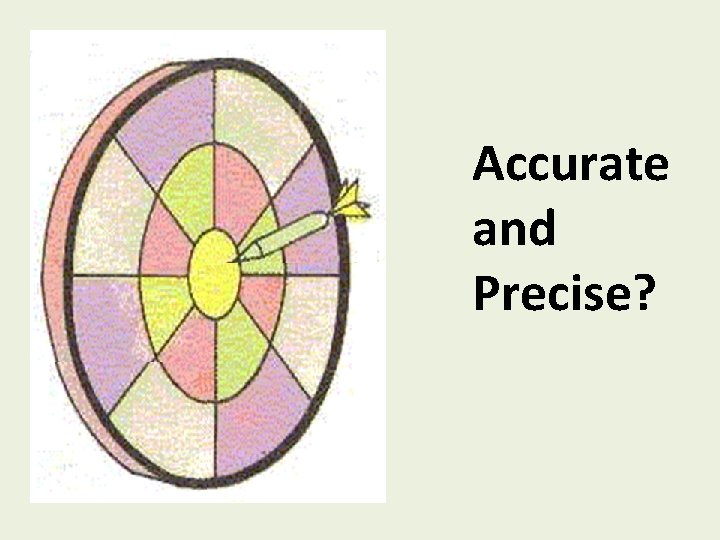
Accurate and Precise

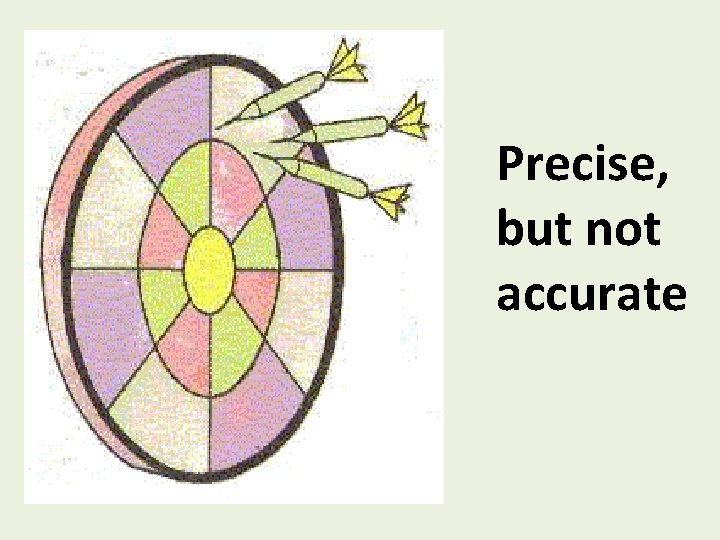
Precise, but not accurate

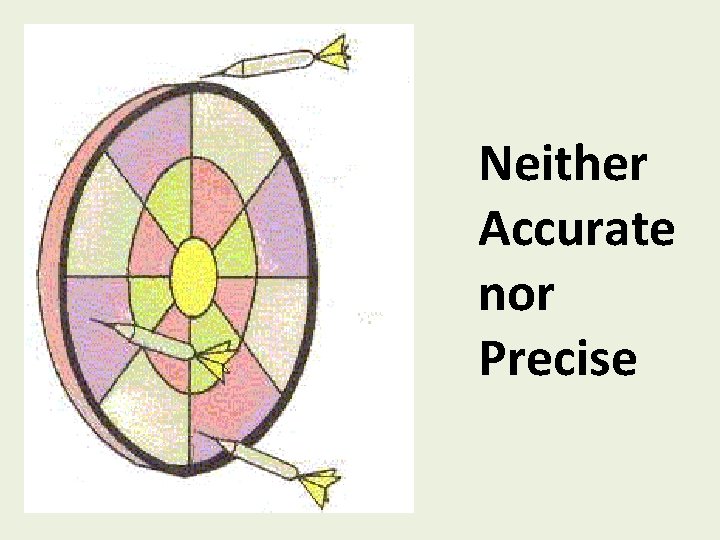
Neither Accurate nor Precise

Accurate and Precise?

Lets Collect Some Class Data How’s your aim?


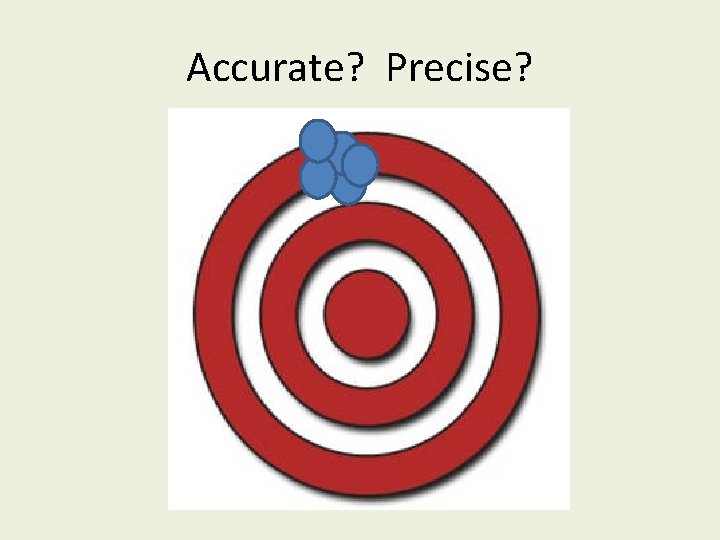
Accurate? Precise?

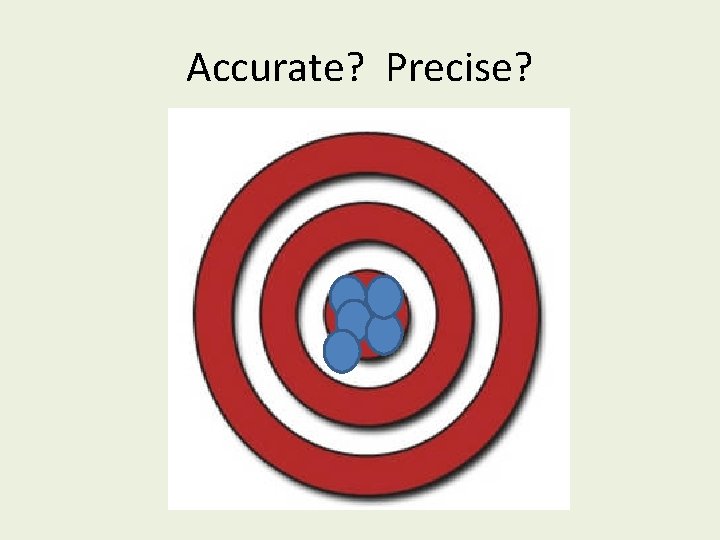
Accurate? Precise?

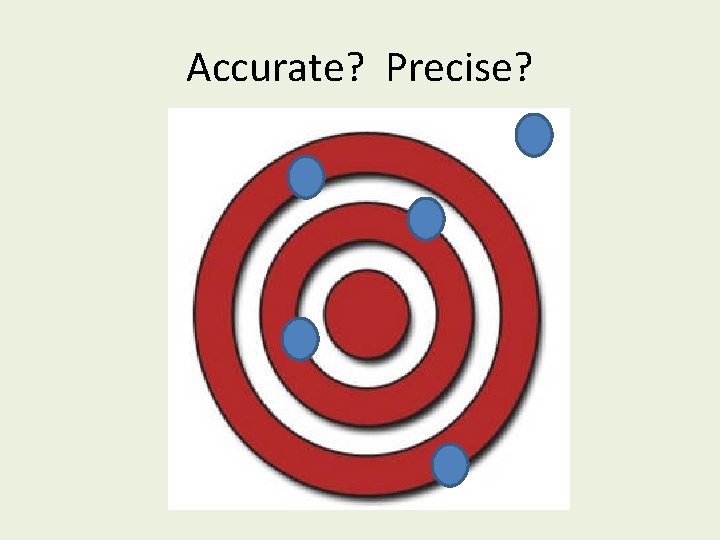
Accurate? Precise?

Accurate? Precise?

EXPERIMENTAL ERROR AND PERCENTAGE ERROR

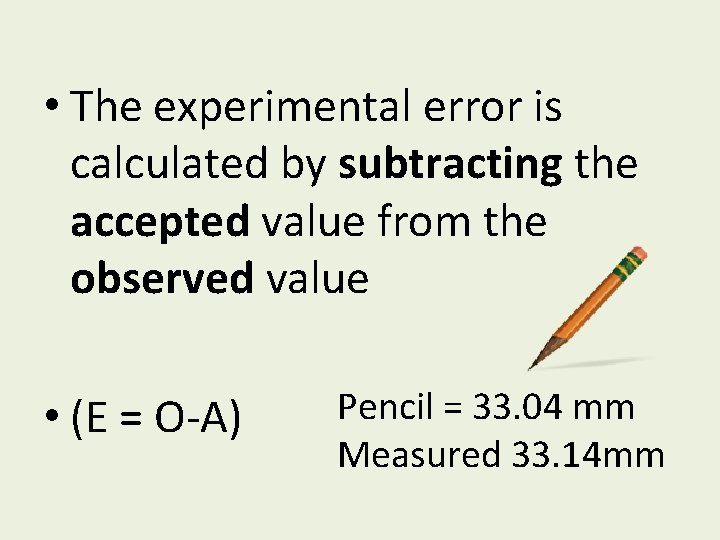
• The experimental error is calculated by subtracting the accepted value from the observed value • (E = O-A) Pencil = 33. 04 mm Measured 33. 14 mm

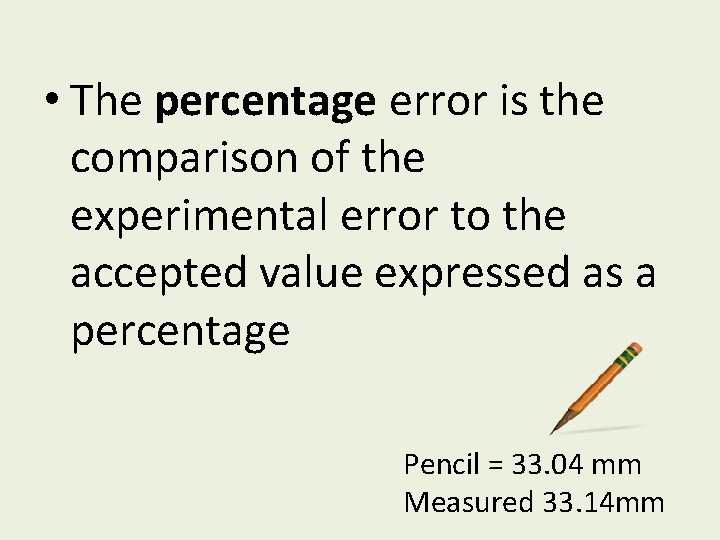
• The percentage error is the comparison of the experimental error to the accepted value expressed as a percentage Pencil = 33. 04 mm Measured 33. 14 mm

• The error may be either positive (the experimental result is too high) or negative (the experimental result is too low) Pencil = 33. 04 mm Measured 32. 99 mm Measured 33. 14 mm

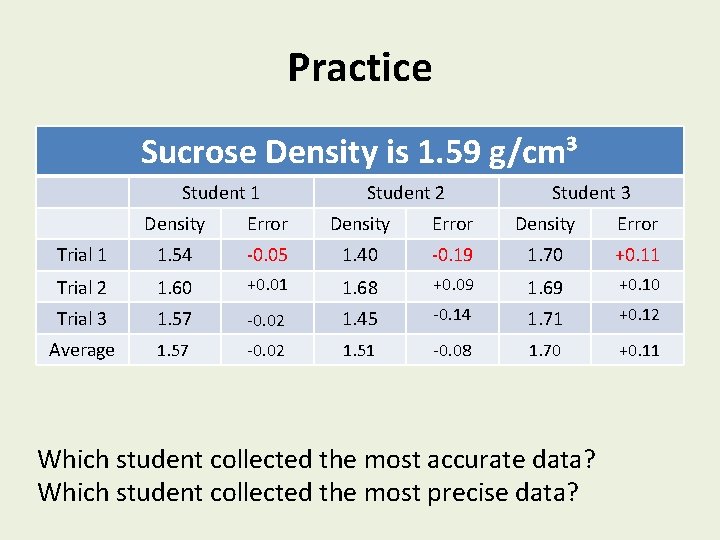
Practice Sucrose Density is 1. 59 g/cm³ Student 1 Student 2 Student 3 Density Error Trial 1 1. 54 -0. 05 1. 40 -0. 19 1. 70 +0. 11 Trial 2 1. 60 +0. 01 1. 68 +0. 09 1. 69 +0. 10 Trial 3 1. 57 -0. 02 1. 45 -0. 14 1. 71 +0. 12 Average 1. 57 -0. 02 1. 51 -0. 08 1. 70 +0. 11 Which student collected the most accurate data? Which student collected the most precise data?

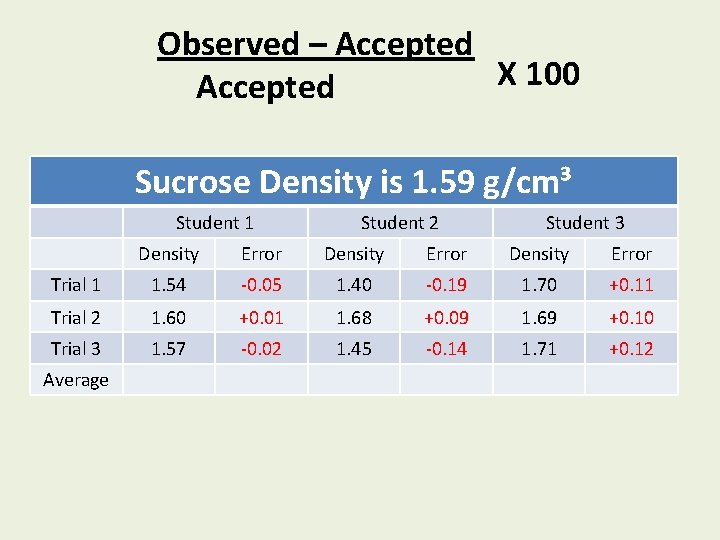
% Error = Observed – Accepted X 100 Accepted Is it positive or negative?

Observed – Accepted X 100 Accepted Sucrose Density is 1. 59 g/cm³ Student 1 Student 2 Student 3 Density Error Trial 1 1. 54 -0. 05 1. 40 -0. 19 1. 70 +0. 11 Trial 2 1. 60 +0. 01 1. 68 +0. 09 1. 69 +0. 10 Trial 3 1. 57 -0. 02 1. 45 -0. 14 1. 71 +0. 12 Average

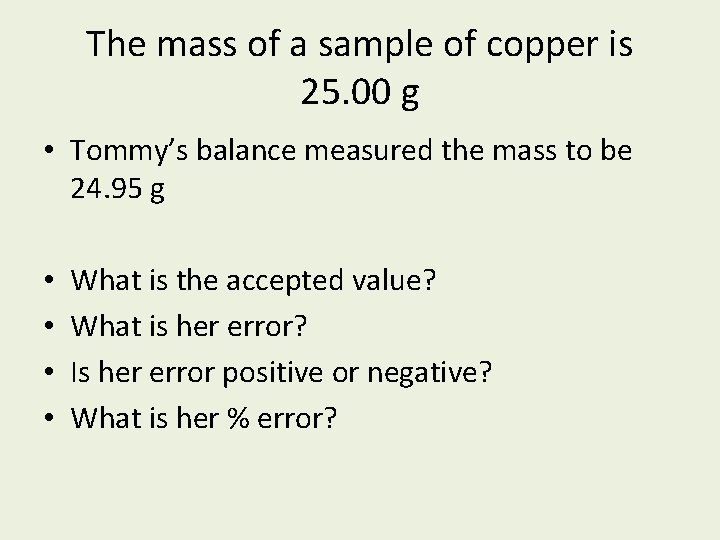
The mass of a sample of copper is 25. 00 g • Tommy’s balance measured the mass to be 24. 95 g • • What is the accepted value? What is her error? Is her error positive or negative? What is her % error?

Significant Numbers

Significant Figures • All the digits in a measurement that can be accurately known plus a last digit that must be estimated

Finding The Number Of Significant Figures In A Number The Easy Rules

If there is a decimal place in the number • Draw an arrow from left to right to find the first non-zero digit, then count the rest of the digits as significant figures

If decimal place… • 25. 0260 = • 0. 00486 =

If there is no decimal place in the number • Draw an arrow from right to left to find the first non-zero digit, then count the rest of the digits as significant figures

If no decimal place… • 250260 = • 13000 =

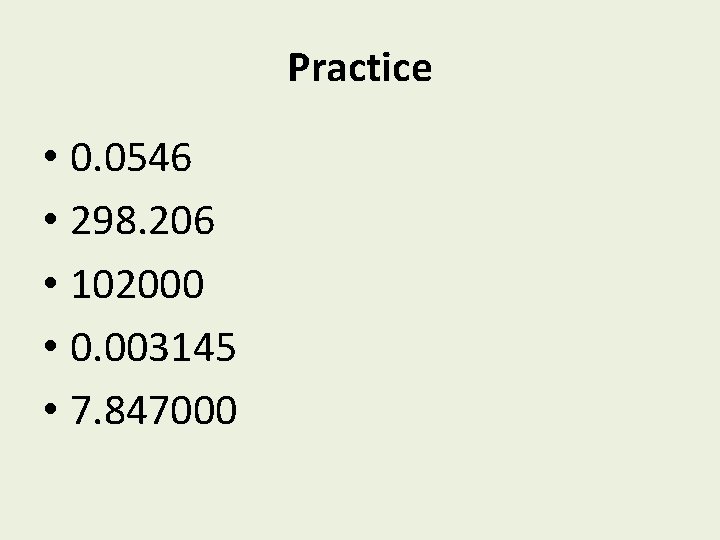
Practice • 0. 0546 • 298. 206 • 102000 • 0. 003145 • 7. 847000

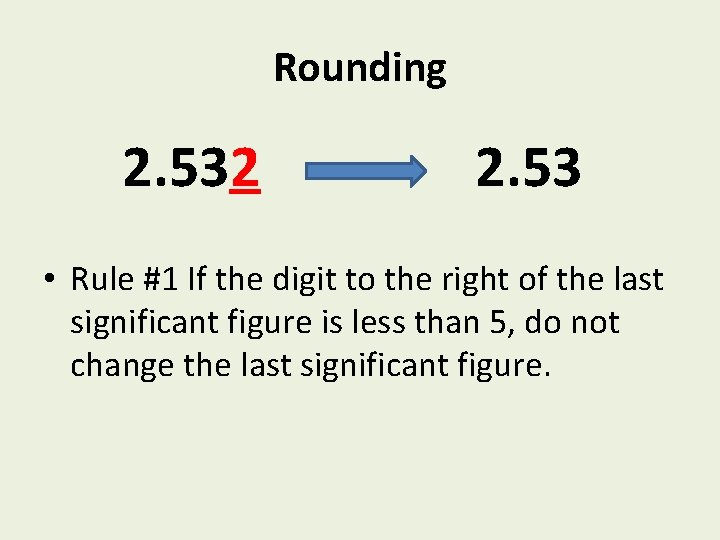
Rounding 2. 532 2. 53 • Rule #1 If the digit to the right of the last significant figure is less than 5, do not change the last significant figure.

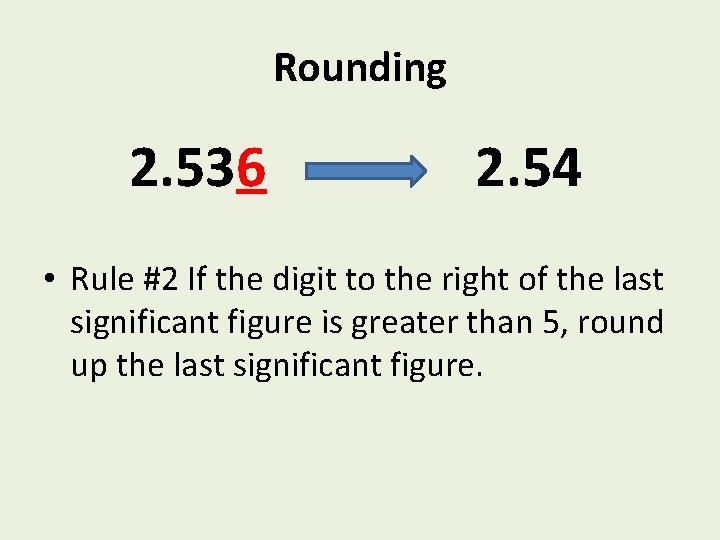
Rounding 2. 536 2. 54 • Rule #2 If the digit to the right of the last significant figure is greater than 5, round up the last significant figure.

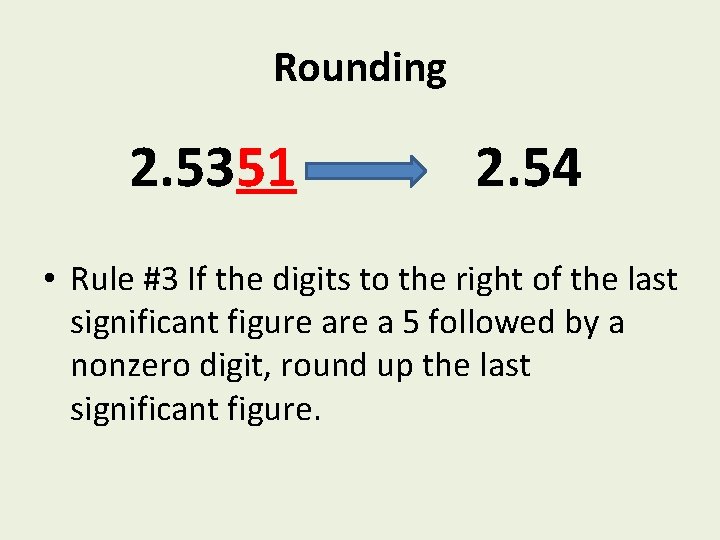
Rounding 2. 5351 2. 54 • Rule #3 If the digits to the right of the last significant figure a 5 followed by a nonzero digit, round up the last significant figure.

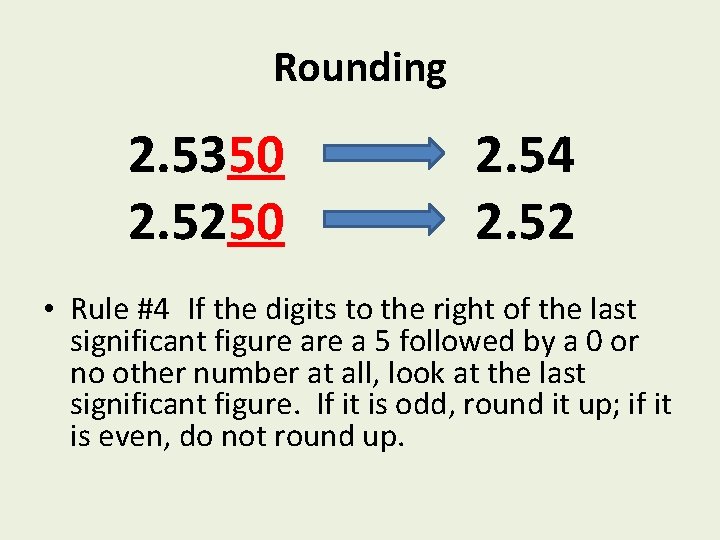
Rounding 2. 5350 2. 5250 2. 54 2. 52 • Rule #4 If the digits to the right of the last significant figure a 5 followed by a 0 or no other number at all, look at the last significant figure. If it is odd, round it up; if it is even, do not round up.

Round each number to four significant figures • 84, 791 • 38. 5432 • 256. 75 • 4. 9356

Round each number to four significant figures, and write the answer in scientific notation • 0. 00054818 • 136, 758 • 308, 659, 000 • 2. 0145