Uncertainties in AMS mass concentrations Especially an important

- Slides: 9

Uncertainties in AMS mass concentrations Especially an important consideration when presenting AMS data to those outside the users’ community! Roya Bahreini AMS Clinic 2012

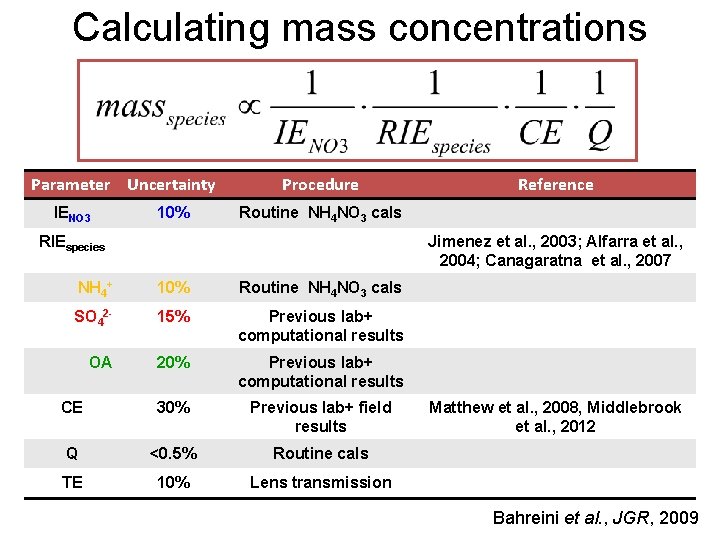
Calculating mass concentrations Parameter Uncertainty IENO 3 10% Procedure Reference Routine NH 4 NO 3 cals RIEspecies Jimenez et al. , 2003; Alfarra et al. , 2004; Canagaratna et al. , 2007 NH 4+ 10% Routine NH 4 NO 3 cals SO 42 - 15% Previous lab+ computational results OA 20% Previous lab+ computational results CE 30% Previous lab+ field results Q <0. 5% Routine cals TE 10% Lens transmission Matthew et al. , 2008, Middlebrook et al. , 2012 Bahreini et al. , JGR, 2009

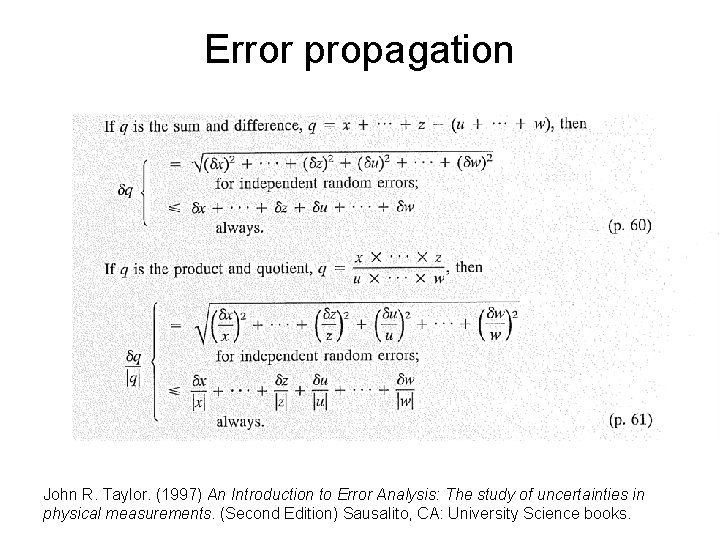
Error propagation John R. Taylor. (1997) An Introduction to Error Analysis: The study of uncertainties in physical measurements. (Second Edition) Sausalito, CA: University Science books.

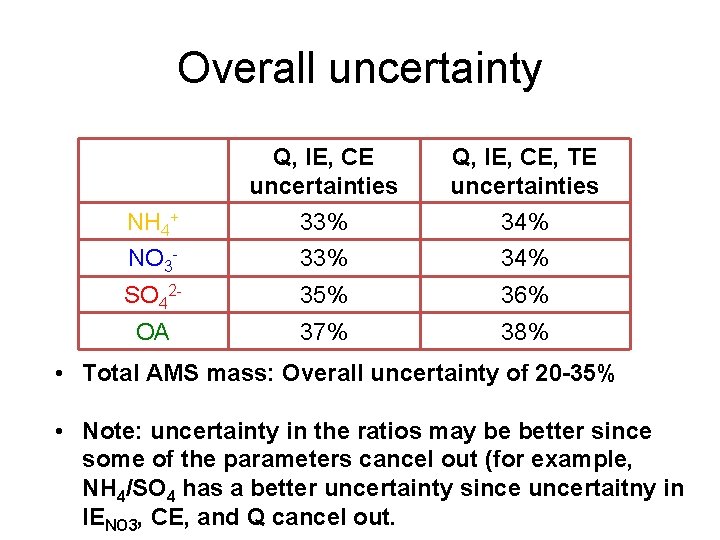
Overall uncertainty Q, IE, CE uncertainties Q, IE, CE, TE uncertainties NH 4+ 33% 34% NO 3 - 33% 34% SO 42 - 35% 36% OA 37% 38% • Total AMS mass: Overall uncertainty of 20 -35% • Note: uncertainty in the ratios may be better since some of the parameters cancel out (for example, NH 4/SO 4 has a better uncertainty since uncertaitny in IENO 3, CE, and Q cancel out.

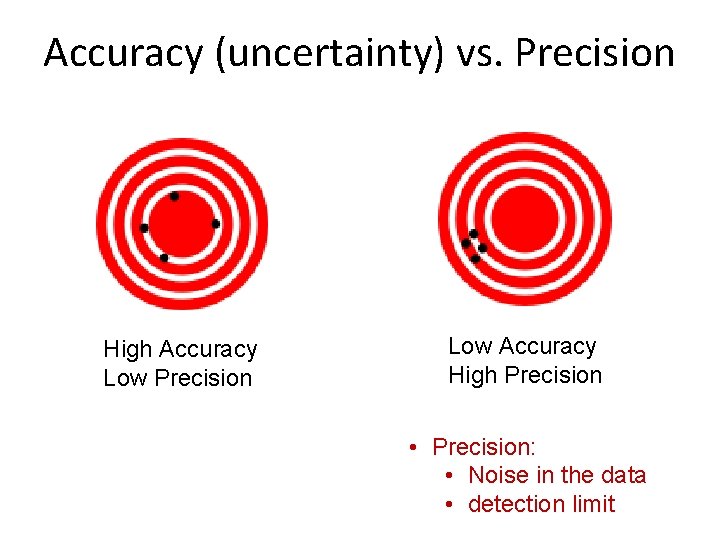
Accuracy (uncertainty) vs. Precision High Accuracy Low Precision Low Accuracy High Precision • Precision: • Noise in the data • detection limit

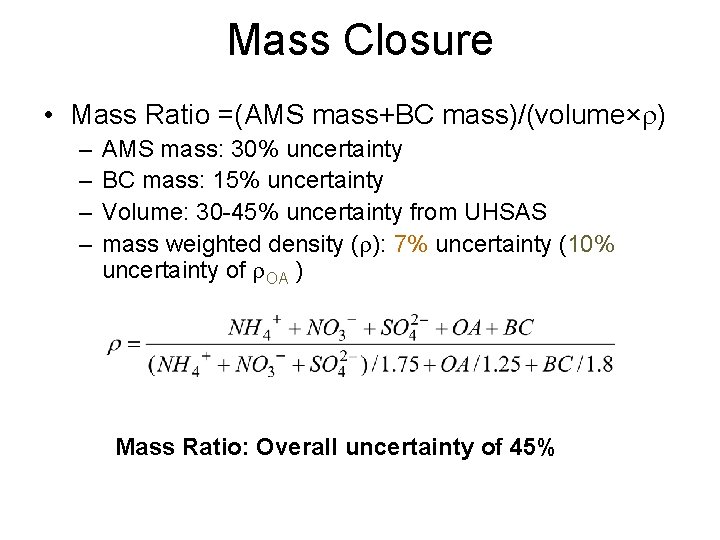
Mass Closure • Mass Ratio =(AMS mass+BC mass)/(volume×r) – – AMS mass: 30% uncertainty BC mass: 15% uncertainty Volume: 30 -45% uncertainty from UHSAS mass weighted density (r): 7% uncertainty (10% uncertainty of r. OA ) Mass Ratio: Overall uncertainty of 45%

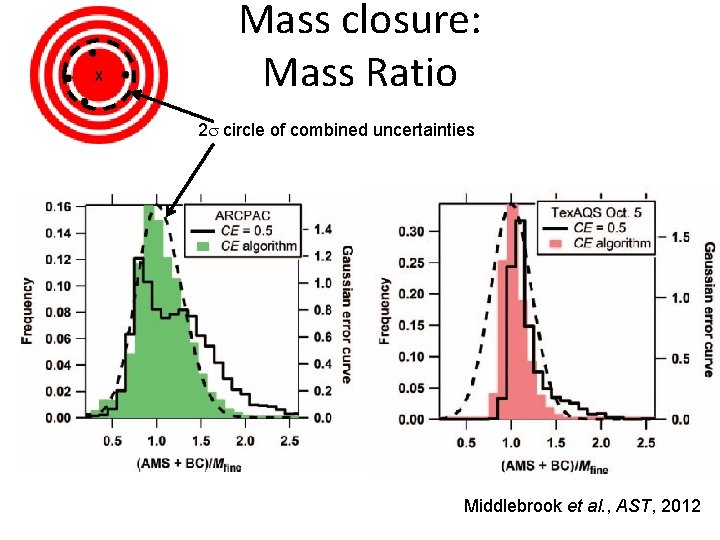
x Mass closure: Mass Ratio 2 s circle of combined uncertainties Middlebrook et al. , AST, 2012

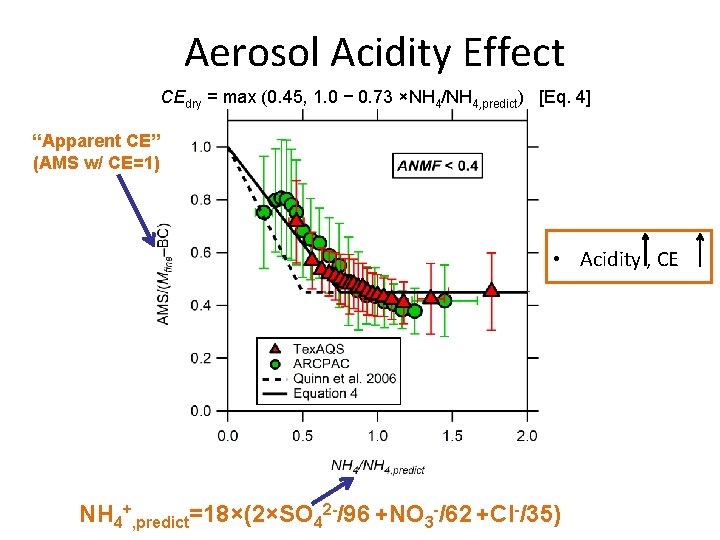
Aerosol Acidity Effect CEdry = max (0. 45, 1. 0 − 0. 73 ×NH 4/NH 4, predict) [Eq. 4] “Apparent CE” (AMS w/ CE=1) • Acidity , CE NH 4+, predict=18×(2×SO 42 -/96 +NO 3 -/62 +Cl-/35)

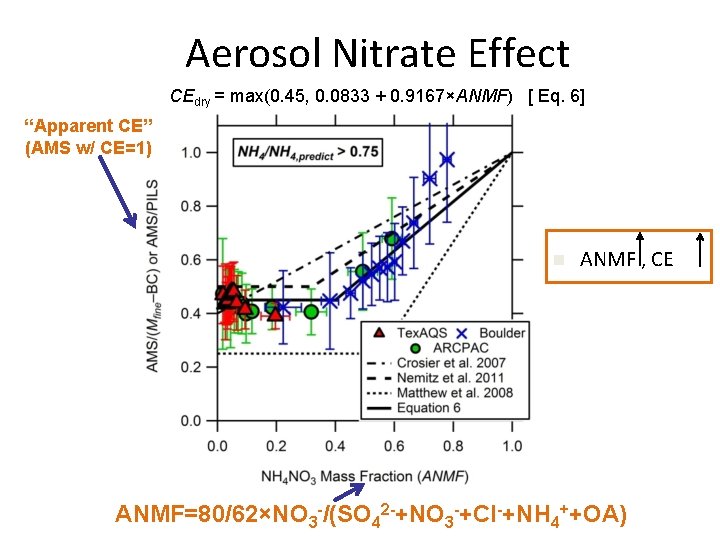
Aerosol Nitrate Effect CEdry = max(0. 45, 0. 0833 + 0. 9167×ANMF) [ Eq. 6] “Apparent CE” (AMS w/ CE=1) n ANMF , CE ANMF=80/62×NO 3 -/(SO 42 -+NO 3 -+Cl-+NH 4++OA)