UMKC Animal Users Meeting SEPT 2019 Introductions UMKC


































- Slides: 38

UMKC Animal Users Meeting SEPT. 2019

Introductions UMKC Vice Chancellor for Research - Dr. Liu (attending Session 1) UMKC Attending Veterinarian – Dr. Scott Korte UMKC IACUC Chair – Dr. Mark Johnson UMKC Biosafety Officer – Jennifer Smith Director of Research Compliance – Chris Winders LARC Director – Jodi Troup IACUC/IBC Compliance Officer – Lori Reierson

• UMKC - member of AAALAC since 1979 at a Full Accreditation Status • AAALAC conducts a site visit every 3 yrs • 1 day visit at UMKC (usually July) • Last visit: July 12 th, 2017 • Visit is based on the Program Description we submit to AAALAC in March of the site visit year. • The Program Description is a detailed account of all UMKC animal related activities; LARC, IACUC and research labs/protocols • Website: https: //www. aaalac. org/

2020 §All day visit §Tour the LARC §Visit some of the PI labs §Lunch with the IACUC §Review IACUC documents (policies, protocols, reports, etc. ) §Exit interview §Summary of finding § Suggestions for improvement § Mandatory corrections Possible results Continued Full Accreditation Conditional Accreditation Deferred Accreditation Probation Revoked Accreditation

FY 20 LARC Per diems

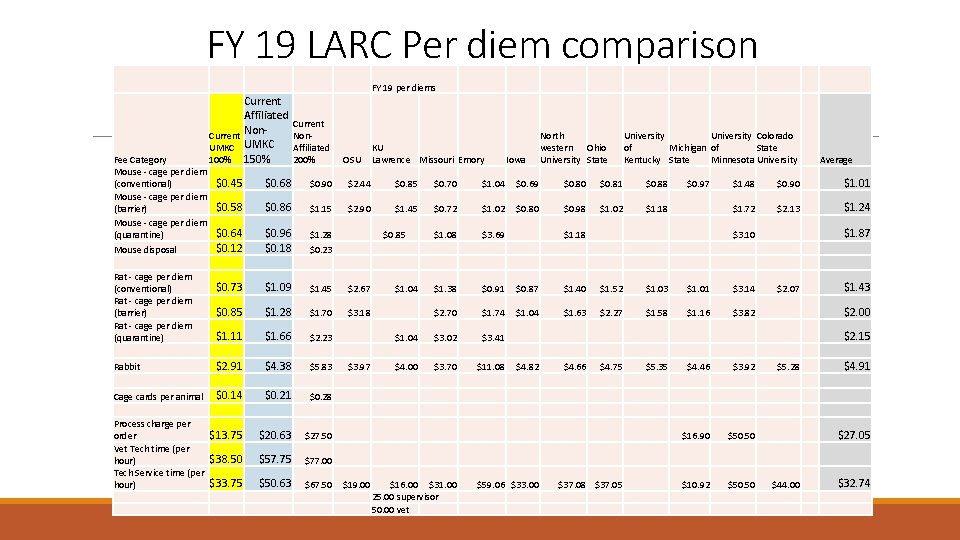
FY 19 LARC Per diem comparison FY 19 per diems Current Affiliated Current Non. UMKC Affiliated KU 100% 150% 200% OSU Lawrence Missouri Emory Fee Category Mouse - cage per diem $0. 45 (conventional) Mouse - cage per diem $0. 58 (barrier) Mouse - cage per diem $0. 64 (quarantine) $0. 12 Mouse disposal Rat - cage per diem $0. 73 (conventional) Rat - cage per diem $0. 85 (barrier) Rat - cage per diem $1. 11 (quarantine) $2. 91 Rabbit Cage cards per animal $0. 14 Process charge per $13. 75 order Vet Tech time (per $38. 50 hour) Tech Service time (per $33. 75 hour) Iowa North western Ohio University State University Colorado of Michigan of State Kentucky State Minnesota University $0. 68 $0. 90 $2. 44 $0. 85 $0. 70 $1. 04 $0. 69 $0. 80 $0. 81 $0. 88 $0. 86 $1. 15 $2. 90 $1. 45 $0. 72 $1. 02 $0. 80 $0. 98 $1. 02 $1. 18 $0. 96 $0. 18 $1. 28 $0. 23 $1. 08 $3. 69 $0. 85 $1. 09 $1. 45 $2. 67 $1. 28 $1. 70 $3. 18 $1. 66 $4. 38 $0. 21 $2. 23 $5. 83 $0. 28 $1. 04 $1. 72 $2. 13 $1. 24 $1. 87 $3. 10 $1. 43 $3. 82 $2. 00 $5. 28 $50. 50 $1. 52 $1. 03 $1. 01 $3. 14 $2. 70 $1. 74 $1. 04 $1. 63 $2. 27 $1. 58 $1. 16 $3. 02 $3. 70 $4. 66 $4. 75 $5. 35 $27. 50 $57. 75 $77. 00 $50. 63 $67. 50 $16. 00 $31. 00 25. 00 supervisor 50. 00 vet $59. 06 $33. 00 $37. 08 $37. 05 $4. 46 $3. 92 $16. 90 $10. 92 $2. 07 $1. 40 $20. 63 $19. 00 $1. 01 $0. 87 $4. 00 $0. 90 $0. 91 $3. 97 $1. 48 $1. 38 $3. 41 $11. 08 $4. 82 $1. 18 $0. 97 Average $50. 50 $44. 00 $2. 15 $4. 91 $27. 05 $32. 74

FY 2020 per diems (effective July 1, 2019) Fees Category UMKC FY 20 Affiliated FY 20 Non-Affiliated FY 20 Mouse - cage per diem (conventional) $0. 46 $0. 69 $0. 93 Mouse - cage per diem (barrier) $0. 59 $0. 88 $1. 18 Mouse - cage per diem (quarantine) $0. 65 $0. 99 $1. 31 Mouse disposal Rabbit - cage per diem Rabbit disposal Rat - cage per diem (conventional) Rat - cage per diem (barrier) Rat - cage per diem (quarantine) Rat disposal Cage cards per animal Process charges per order Vet Tech Service Time (per hr) Additional supplies (cost + %) Radil testing (cost + %) $0. 12 $3. 00 $3. 90 $0. 74 $0. 87 $1. 15 $0. 84 $0. 15 $14. 16 $39. 66 $34. 76 20% $0. 18 $4. 51 $5. 86 $1. 12 $1. 31 $1. 71 $1. 26 $0. 22 $21. 24 $59. 48 $52. 14 25% $0. 23 $6. 00 $7. 80 $1. 49 $1. 75 $2. 29 $1. 68 $0. 28 $28. 33 $79. 31 $69. 52 25% In FY 16 our mouse cage per diem was $0. 36/cage. We had an 8. 33% increase in FY 17, FY 18 and FY 19. Starting FY 20 there will be a 3% annual increase in per diems.

Husbandry costs increases Teklad feed: ◦ ◦ FY 16: $26. 65/bag + $16. 00 shipping FY 17: $26. 65/bag + $16. 00 shipping FY 18: $27. 40/bag + $16. 00 shipping FY 19: $32. 30/bag + $55. 00 shipping Teklad bedding: ◦ FY 16: $15. 70/bag + $16. 00 shipping ◦ FY 17: $16. 20/bag + $16. 00 shipping ◦ FY 18: $17. 00/ bag + $16. 00 shipping ◦ FY 19: $19. 10/bag + $55. 00 shipping Average annual cost: $19, 031. 52/yr Average annual cost: $11, 167. 43/yr

IACUC Review Timelines

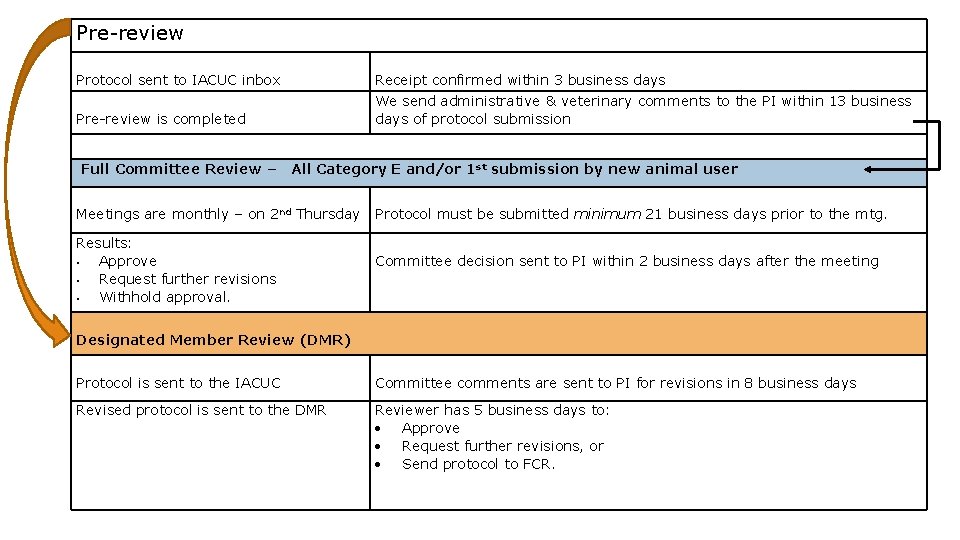
Pre-review Protocol sent to IACUC inbox Receipt confirmed within 3 business days Pre-review is completed We send administrative & veterinary comments to the PI within 13 business days of protocol submission Full Committee Review – All Category E and/or 1 st submission by new animal user Meetings are monthly – on 2 nd Thursday Protocol must be submitted minimum 21 business days prior to the mtg. Results: • Approve • Request further revisions • Withhold approval. Committee decision sent to PI within 2 business days after the meeting Designated Member Review (DMR) Protocol is sent to the IACUC Committee comments are sent to PI for revisions in 8 business days Revised protocol is sent to the DMR Reviewer has 5 business days to: Approve Request further revisions, or Send protocol to FCR.

IACUC Reviewing Activity for 2018 -19 IACUC currently has 65 approved protocols Reviewing Activity for July 2018 -June 2019 9 new protocol submissions 15 Three Year renewals 24 annual continuations 47 amendments (major and minor)

New Tumor Burden Policy All IACUC SOPs are posted on the IACUC webpage Ø SSO log in is required Ø Humane Endpoints for Studies Involving Spontaneous and Induced Tumors If you encounter any difficulties with the website, contact Lori Reierson at 816 -235 -5929 or umkciacuc@umkc. edu

Trainings The IACUC chair is available to host a training session on cervical dislocation. Brand new to the technique or would like to refresh your skills? Please send your training request to UMKCIACUC@umkc. edu and we will organize the session as soon as possible.

Reporting Injuries Every person working with animals should be aware of the potential for injuries and accidents (i. e. animal bites, needle sticks). Always report injuries and accidents. Seek emergency first aid. Notify your supervisor Notify the LARC Director Notify Environmental Health & Safety and/or UMKC Biosafety Officer Campus Police: 816 -235 -1515 816 -235 -5669 816 -235 -5421 816 -235 -1844 § Complete a “Report of Injury” form and submit it to UMKC workers compensation coordinator

Controlled Substances at UMKC REGISTRATION, PURCHASE, STORAGE, AND DISPOSAL

Background The Federal Drug Enforcement Administration (DEA) and the Missouri Bureau of Narcotics and Dangerous Drugs (BNDD) require that certain substances that can be abused be registered with both agencies Controlled substances are placed in Schedules (I-V) depending upon their potential for medical use and potential for abuse All UMKC Principal Investigators dispensing or administering controlled substances as part of their research program must obtain a Research Registration from the DEA and the BNDD DEA and BNDD registration is FREE for State/Federal employees The information in this presentation is provided as a courtesy and may not be updated regularly. It is the Principal Investigator’s responsibility to ensure compliance with State and Federal Regulations. The Principal Investigator maintains all responsibility for the purchase, storage, dispensing and administration of controlled substances under their registrations

Schedules I-V Schedule I Controlled Substances ◦ Substances in this schedule have no currently accepted medical use in the United States, a lack of accepted safety for use under medical supervision, and a high potential for abuse. ◦ Some examples of substances listed in Schedule I are: heroin, lysergic acid diethylamide (LSD), marijuana (cannabis), peyote, methaqualone, and 3, 4 -methylenedioxymethamphetamine ("Ecstasy"). Schedule II/IIN Controlled Substances (2/2 N) ◦ Substances in this schedule have a high potential for abuse which may lead to severe psychological or physical dependence. ◦ Examples of Schedule II narcotics include: hydromorphone (Dilaudid®), methadone (Dolophine®), meperidine (Demerol®), oxycodone (Oxy. Contin®, Percocet®), and fentanyl (Sublimaze®, Duragesic®). ◦ Other Schedule II narcotics include: morphine, opium, codeine, and hydrocodone. ◦ Examples of Schedule IIN stimulants include: amphetamine (Dexedrine®, Adderall®), methamphetamine (Desoxyn®), and methylphenidate (Ritalin®). Other Schedule II substances include: amobarbital, glutethimide, and pentobarbital.

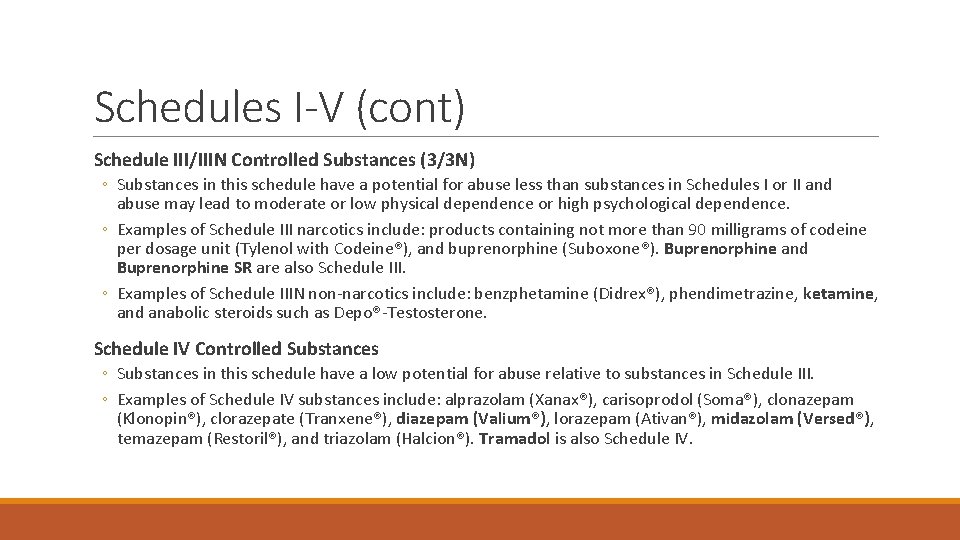
Schedules I-V (cont) Schedule III/IIIN Controlled Substances (3/3 N) ◦ Substances in this schedule have a potential for abuse less than substances in Schedules I or II and abuse may lead to moderate or low physical dependence or high psychological dependence. ◦ Examples of Schedule III narcotics include: products containing not more than 90 milligrams of codeine per dosage unit (Tylenol with Codeine®), and buprenorphine (Suboxone®). Buprenorphine and Buprenorphine SR are also Schedule III. ◦ Examples of Schedule IIIN non-narcotics include: benzphetamine (Didrex®), phendimetrazine, ketamine, and anabolic steroids such as Depo®-Testosterone. Schedule IV Controlled Substances ◦ Substances in this schedule have a low potential for abuse relative to substances in Schedule III. ◦ Examples of Schedule IV substances include: alprazolam (Xanax®), carisoprodol (Soma®), clonazepam (Klonopin®), clorazepate (Tranxene®), diazepam (Valium®), lorazepam (Ativan®), midazolam (Versed®), temazepam (Restoril®), and triazolam (Halcion®). Tramadol is also Schedule IV.

Schedules I-V (cont) Schedule V Controlled Substances ◦ Substances in this schedule have a low potential for abuse relative to substances listed in Schedule IV and consist primarily of preparations containing limited quantities of certain narcotics. ◦ Examples of Schedule V substances include: cough preparations containing not more than 200 milligrams of codeine per 100 milliliters or per 100 grams (Robitussin AC®, Phenergan with Codeine®), and ezogabine.

Application Process – BNDD Bureau of Narcotics and Dangerous Drugs (State of Missouri) ◦ ◦ State version of the DEA Application MUST BE FILLED OUT AND APPROVED BEFORE DEA APPLICATION https: //health. mo. gov/safety/bndd/ The BNDD does notify you of your successful application or renewal deadlines ◦ Must go to the BNDD website and click on the “Print a Registration Certificate or Verify a Registration” link to find your BNDD Registration number ◦ You will need to know which controlled substances you plan on administering and their schedules during the application process

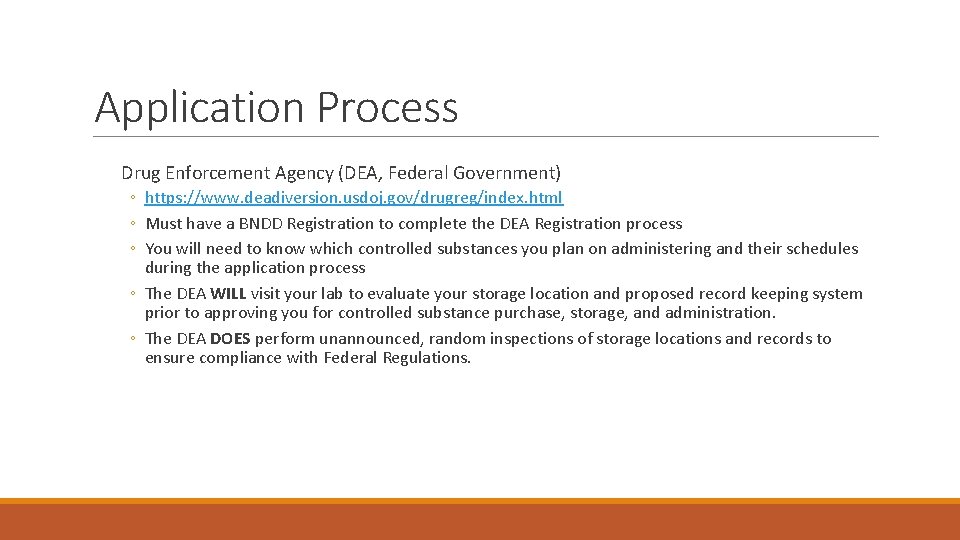
Application Process Drug Enforcement Agency (DEA, Federal Government) ◦ https: //www. deadiversion. usdoj. gov/drugreg/index. html ◦ Must have a BNDD Registration to complete the DEA Registration process ◦ You will need to know which controlled substances you plan on administering and their schedules during the application process ◦ The DEA WILL visit your lab to evaluate your storage location and proposed record keeping system prior to approving you for controlled substance purchase, storage, and administration. ◦ The DEA DOES perform unannounced, random inspections of storage locations and records to ensure compliance with Federal Regulations.

Storage Requirements Securely locked, substantially constructed cabinet or safe Key or combination access restricted to those individuals listed on your registration Safe or cabinet must be locked at all times, unless accessing controlled substances If the safe is small or portable it must be bolted to the floor or wall or placed inside another locked cabinet Each registrant must have their own individual, locked controlled substance storage ◦ (would really prefer all controlled substances behind 2 locks with different keys or combinations) Only individuals listed on the Registration can access controlled substances

Record keeping Purchasing/Receipt Records Initial Inventory Annual Inventory Administration/Dispensing Disposal of Unwanted Substances Reporting Loss Audits Schedule II substance records must be maintained separately from Schedule III-V

Purchasing and Receiving records Maintain the original invoice/receipt with suppliers: ◦ Name, address, and DEA number of supplier Must also maintain receiving record with: ◦ Name, address, and DEA number of recipient ◦ Drug name, strength, form, and quantities received ◦ Date of receipt



Initial Inventory Very first day that you receive controlled substances Must include: ◦ ◦ Registrants name and DEA number Date Drug name, strength, dose form, and quantities Time of day the inventory was taken (opening or closing of business) May use Annual Inventory Form, denoting that it is the Initial Inventory


Perpetual Inventory Ongoing, accurate, up-to-date total of substances administered Not required, but it is the easiest mechanism to demonstrated the administration of your controlled substances. Should you be audited by the DEA or BNDD, you will have to demonstrate where all of your controlled substances have been administered. A perpetual inventory is easier to review than your research records for that information.


Annual Inventories Must include: ◦ ◦ Registrants name and DEA number Date Drug name, strength, dose form, and quantities Time of day the inventory was taken (opening or closing of business) Must be conducted annually (BNDD) ◦ Every 2 years for DEA


Disposal Excess, waste, or expired controlled substances must be disposed of appropriately ◦ Cannot be put down the sink or into sharps containers ◦ Injection into carcasses for incineration is acceptable Disposal must be recorded on the DEA Form 41 and the disposal must be witnessed by 2 other individuals


Theft/Loss (AKA Diversion) Upon discovery of theft or significant loss, Registrants must report the loss, in writing, to the DEA using DEA Form 106 ◦ https: //apps 2. deadiversion. usdoj. gov/TLR/login. xhtml ◦ Requires last name and DEA registration number to login MUST BE REPORTED WITHIN ONE BUISNESS DAY

“Significant Loss” 1) The actual quantity of controlled substances lost in relation to the type of business; 2) The specific controlled substances lost; 3) Whether the loss of the controlled substances can be associated with access to those controlled substances by specific individuals, or whether the loss can be attributed to unique activities that may take place involving the controlled substances; 4) A pattern of losses over a specific time period, whether the losses appear to be random, and the results of efforts taken to resolve the losses; and, if known, 5) Whether the specific controlled substances are likely candidates for diversion; and 6) Local trends and other indicators of the diversion potential of the missing controlled substance. https: //www. uspharmacist. com/article/dea-form-106 -and-loss-of-controlled-substances

Records retention All records must be maintained for at least 2 years following completion ◦ BNDD requires annual inventories ◦ DEA requires maintaining 2 biennial inventories at all times ◦ Must be maintained for 2 years past completion Records must be stored in the same location as the controlled substances

Questions are welcome!
 Meeting agenda welcome and introductions
Meeting agenda welcome and introductions Agenda welcome and introductions
Agenda welcome and introductions Meeting agenda welcome and introductions
Meeting agenda welcome and introductions Meeting agenda welcome and introductions
Meeting agenda welcome and introductions Rhic ags users meeting 2020
Rhic ags users meeting 2020 What does the prefix sept mean
What does the prefix sept mean Damon poole
Damon poole La guerre de sept ans
La guerre de sept ans Sept commandements
Sept commandements Sept
Sept Hills of jerusalem
Hills of jerusalem Cnn 10 september 3
Cnn 10 september 3 Une deux trois quatre cinq
Une deux trois quatre cinq I sept
I sept Le blaireau sans gêne poésie
Le blaireau sans gêne poésie Sept heure moins le quart
Sept heure moins le quart Deportes
Deportes Sept comme setteur questionnaire
Sept comme setteur questionnaire Ecrivez les sept jours de la semaine
Ecrivez les sept jours de la semaine Sae government industry meeting 2019
Sae government industry meeting 2019 Nrg oncology meeting 2016
Nrg oncology meeting 2016 Nrg oncology conference
Nrg oncology conference Sae government industry meeting 2019
Sae government industry meeting 2019 Nrg oncology meeting 2018
Nrg oncology meeting 2018 What is meeting and types of meeting
What is meeting and types of meeting What is meeting and types of meeting
What is meeting and types of meeting Today meeting or today's meeting
Today meeting or today's meeting Proposal kickoff meeting agenda
Proposal kickoff meeting agenda Serve2learn
Serve2learn Umkc gme
Umkc gme Umkc online
Umkc online Respondus lockdown browser vt
Respondus lockdown browser vt Interesting introductions
Interesting introductions Agenda welcome and introductions
Agenda welcome and introductions Introductions by marsha
Introductions by marsha Examples of survey introductions
Examples of survey introductions Project introductions
Project introductions Creative introductions
Creative introductions Greetings in english dialogue
Greetings in english dialogue