Ultrafast Spectroscopy Of Methyl Viologen Effects Of Zeolite



- Slides: 13

Ultrafast Spectroscopy Of Methyl Viologen: Effects Of Zeolite Entrapment Joseph Henrich, Haoyu Zhang, Jeremy White, Prabir Dutta and Bern Kohler The Ohio State University Department of Chemistry Molecular Spectroscopy Symposium June 17, 2008

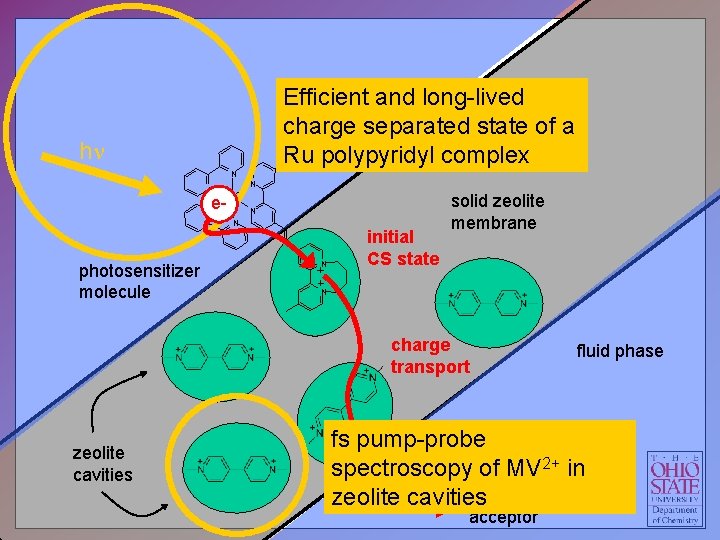
Efficient fluid phaseand long-lived charge separated state of a 2+ polypyridyl complex Ru hn N N e-Ru N N photosensitizer molecule N initial CS state solid zeolite membrane N charge transport zeolite cavities fluid phase fs pump-probe spectroscopy of MV 2+ in Electron zeolite cavities acceptor

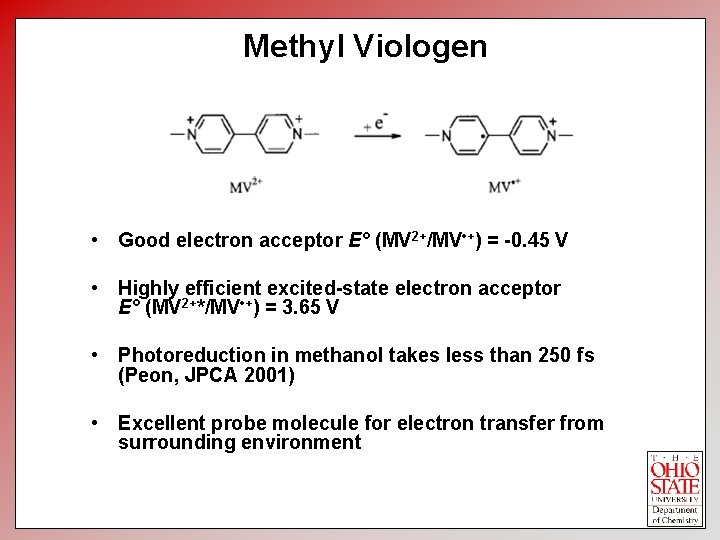
Methyl Viologen • Good electron acceptor E° (MV 2+/MV • +) = -0. 45 V • Highly efficient excited-state electron acceptor E° (MV 2+*/MV • +) = 3. 65 V • Photoreduction in methanol takes less than 250 fs (Peon, JPCA 2001) • Excellent probe molecule for electron transfer from surrounding environment

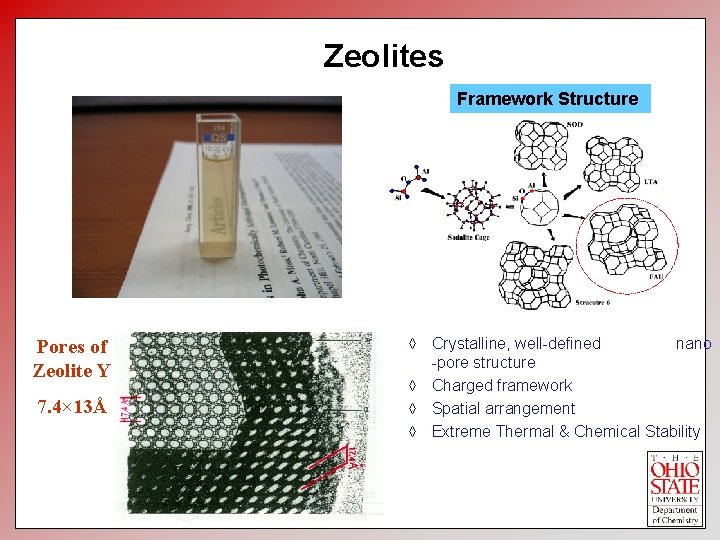
Zeolites Framework Structure Pores of Zeolite Y 7. 4× 13Å à Crystalline, well-defined nano -pore structure à Charged framework à Spatial arrangement à Extreme Thermal & Chemical Stability

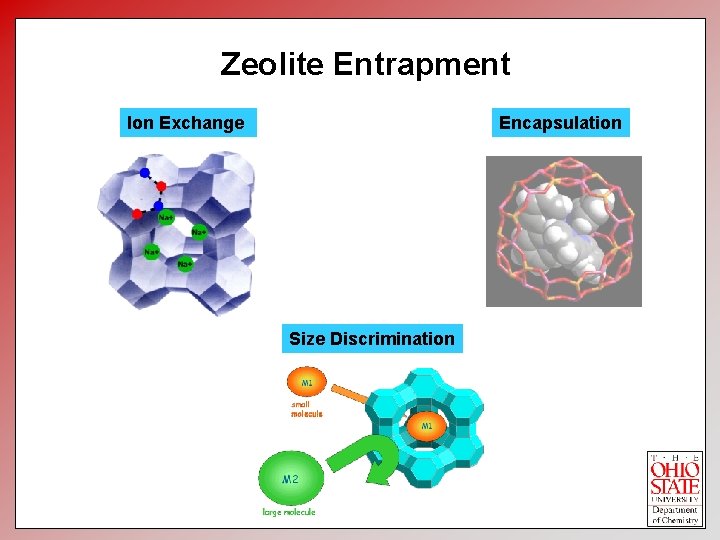
Zeolite Entrapment Encapsulation Ion Exchange Size Discrimination

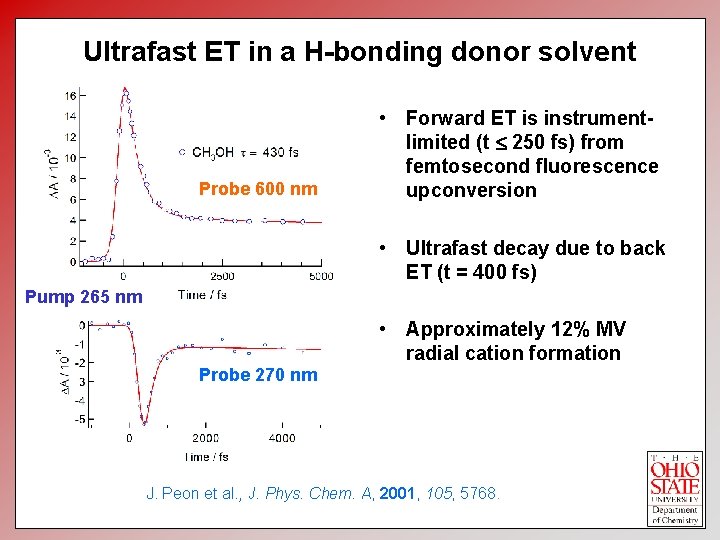
Ultrafast ET in a H-bonding donor solvent Probe 600 nm • Forward ET is instrumentlimited (t 250 fs) from femtosecond fluorescence upconversion • Ultrafast decay due to back ET (t = 400 fs) Pump 265 nm • Approximately 12% MV radial cation formation Probe 270 nm J. Peon et al. , J. Phys. Chem. A, 2001, 105, 5768.

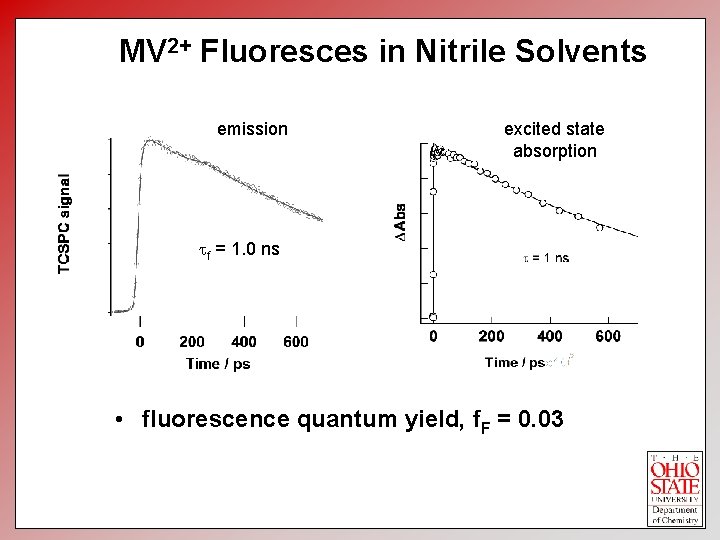
MV 2+ Fluoresces in Nitrile Solvents emission excited state absorption tf = 1. 0 ns • fluorescence quantum yield, f. F = 0. 03

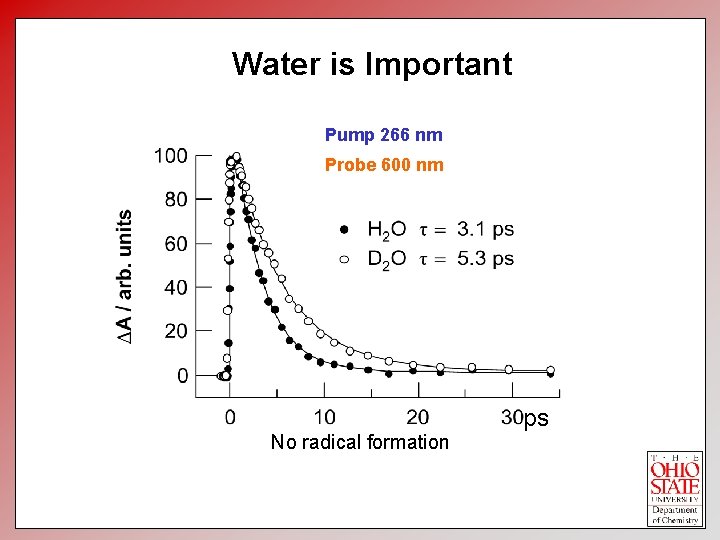
Water is Important Pump 266 nm Probe 600 nm No radical formation ps

MV in Zeolites • Reports of methyl viologen radical formation when entrapped inside of supercages • In Zeolite Y (Scaiano, JCPB 1997) • In Zeolite X (Ranjit, JPCB 2002) zeolites

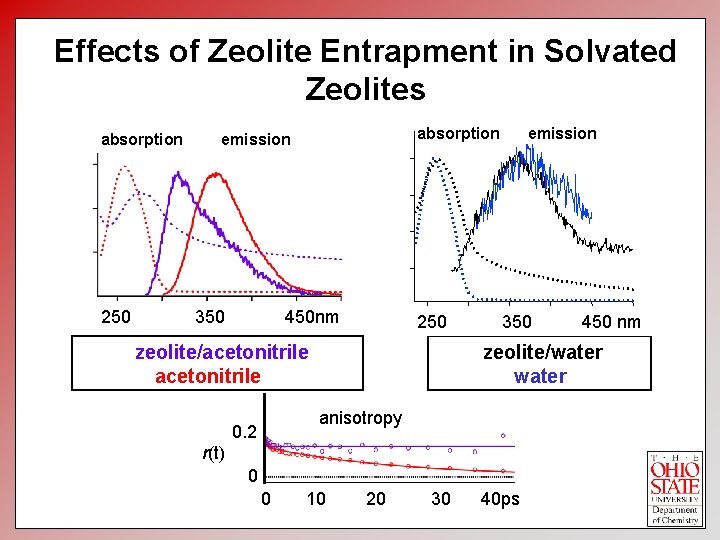
Effects of Zeolite Entrapment in Solvated Zeolites absorption emission Normalized Absorption and Emission absorption 250 350 450 nm 250 zeolite/acetonitrile 300 350 400 Wavelength (nm) 350 zeolite/water anisotropy 0. 2 r(t) 0 0 450 10 20 30 40 ps 500 450 nm

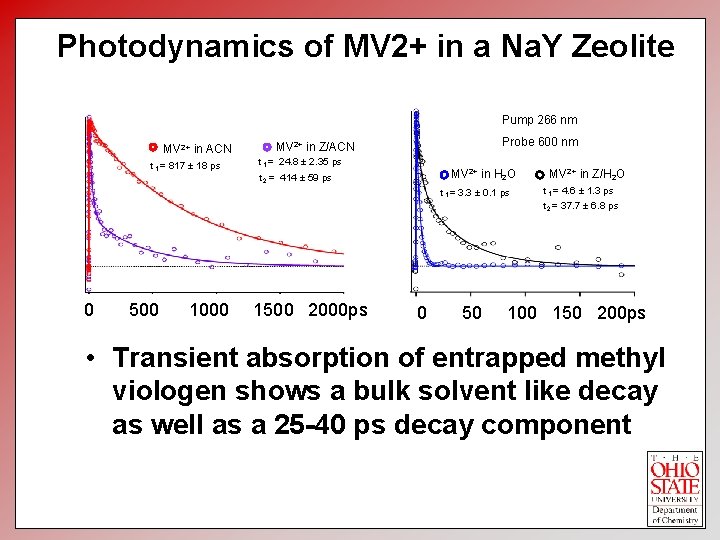
Photodynamics of MV 2+ in a Na. Y Zeolite Pump 266 nm MV 2+ in ACN t 1 = 817 ± 18 ps Probe 600 nm MV 2+ in Z/ACN t 1 = 24. 8 ± 2. 35 ps MV 2+ in H 2 O t 2 = 414 ± 59 ps t 1 = 3. 3 ± 0. 1 ps MV 2+ in Z/H 2 O t 1 = 4. 6 ± 1. 3 ps t 2 = 37. 7 ± 6. 8 ps 0 500 1000 1500 2000 ps 0 50 Time delay 100(ps)150 200 ps • Transient absorption of entrapped methyl viologen shows a bulk solvent like decay as well as a 25 -40 ps decay component

Conclusions • The methyl viologen excited state has distinct decay pathways that are solvent dependent. • Nano-zeolites make it feasible to make traditional pump/probe transient absorption measurements on encapsulated molecules with high signal-to-niose. • When entrapped in zeolites methyl viologen is rigidly held within the supercage. • A new decay process is created due to electron transfer from the zeolite framework to excited-state methyl viologen.

Acknowledgments Dr. Bern Kohler Dr. Prabir Dutta Dr. Haoyu Zhang Jeremy White Kohler Group Funding Department of Energy