UC 5 Assigning of Epoch after treatment discontinuation









- Slides: 16

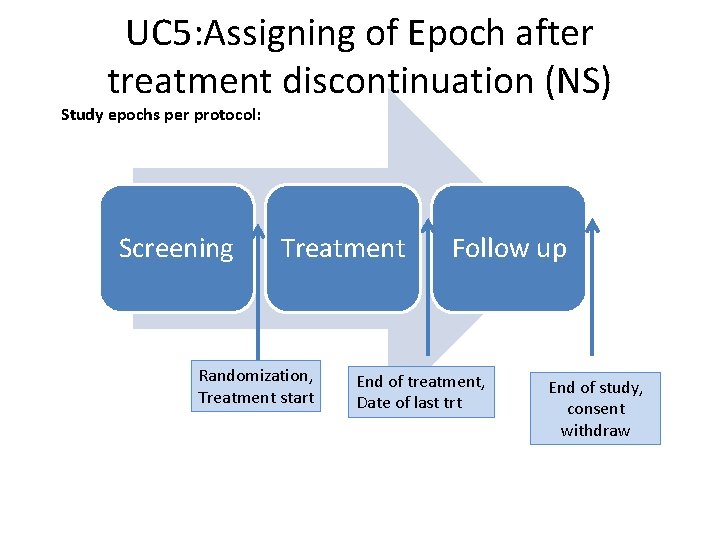
UC 5: Assigning of Epoch after treatment discontinuation (NS) Study epochs per protocol: Screening Treatment Randomization, Treatment start Follow up End of treatment, Date of last trt End of study, consent withdraw

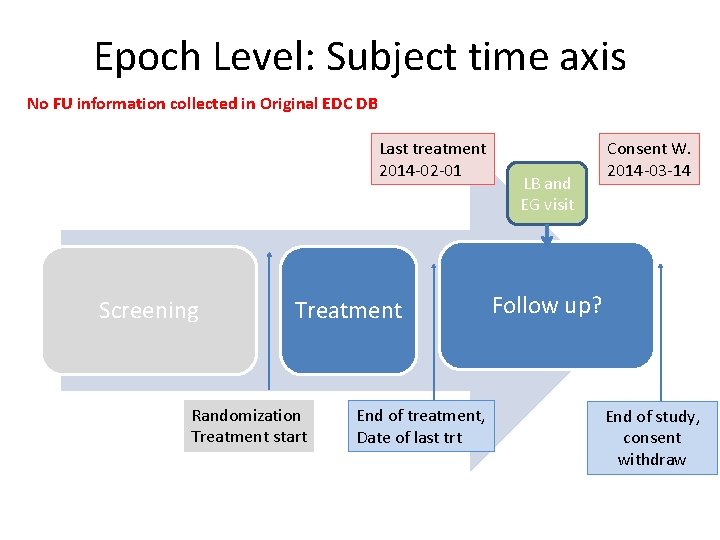
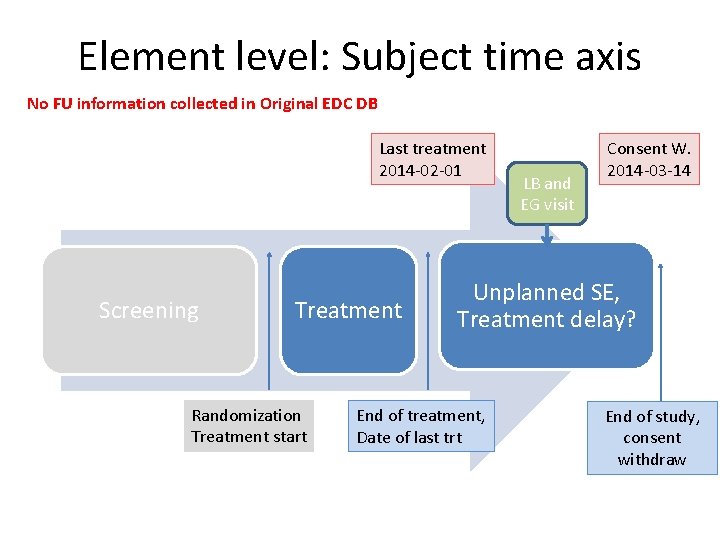
UC 5: (cont. ) subject information • CRF pages: end of treatment, FU, end of study • Subject has got 2 cycles. Last TRTDTC=2014 -02 -01 • Cycle 3 should start at 2014 -02 -21, but subject has AE, thus treatment delay, • No bettering after 14 days, Lab visit, EG Visit performed. => Physician decision to discontinue treatment • Subject comes to visit and decide not to go to FU, but to withdraw consent at 2014 -03 -14, so no FU observation and data collection, end of study. • Q: Which epoch and VISITNUM to assign to LB and EG visit in delay period?

Question • On which level should we handle treatment delay period: EPOCH or ELEMENT?

Definition from CDISC SDTM • Epoch: As part of the design of a trial, the planned period of subjects' participation in the trial is divided into Epochs. Each Epoch is a period of time that serves a purpose in the trial as a whole. That purpose will be at the level of the primary objectives of the trial. Typically, the purpose of an Epoch will be to expose subjects to a treatment, or to prepare for such a treatment period (e. g. , determine subject eligibility, wash out previous treatments) or to gather data on subjects after a treatment has ended.

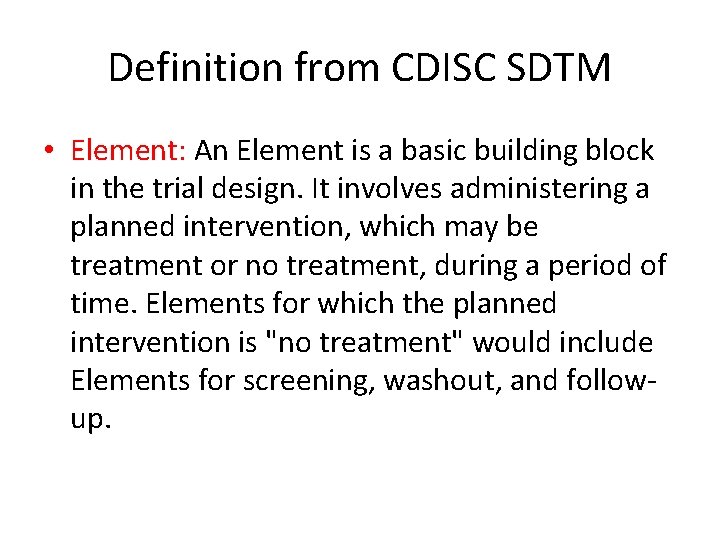
Definition from CDISC SDTM • Element: An Element is a basic building block in the trial design. It involves administering a planned intervention, which may be treatment or no treatment, during a period of time. Elements for which the planned intervention is "no treatment" would include Elements for screening, washout, and followup.

Definition from CDISC SDTM • “If TAETORD and/or EPOCH are added, then the values must be those at the start of the visit” Chapter 5, “SV”, P 3 • 5. Judgment will also have to be used in deciding how to represent a subject's experience if an Element does not proceed or end as planned. For instance, the plan might identify a trial Element which is to start with the first of a series of 5 daily doses and end after 1 week, when the subject transitions to the next treatment Element. If the subject actually started the next treatment Epoch [see Section 7 - Introduction: 7. 1. 2, Definitions Of Trial Design Concepts] after 4 weeks, the sponsor will have to decide whether to represent this as an abnormally long Element, or as a normal Element plus an unplanned non-treatment Element. Chapter 5, “SE”, P 3

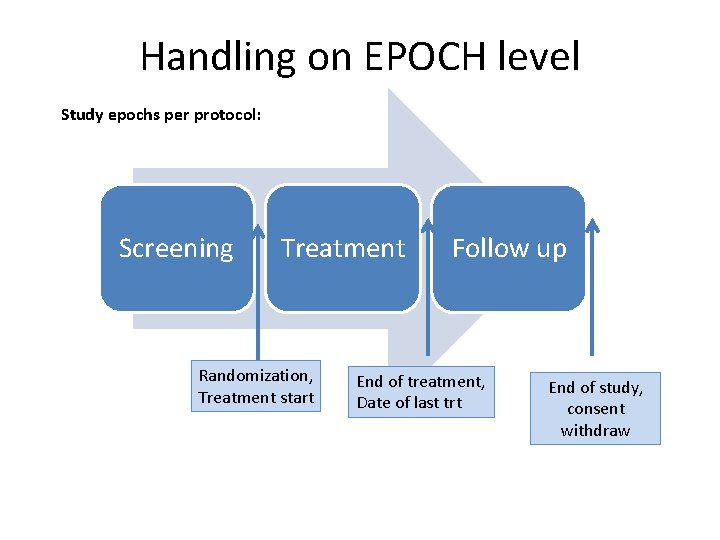
Handling on EPOCH level Study epochs per protocol: Screening Treatment Randomization, Treatment start Follow up End of treatment, Date of last trt End of study, consent withdraw

Epoch Level: Subject time axis No FU information collected in Original EDC DB Last treatment 2014 -02 -01 Screening Treatment Randomization Treatment start End of treatment, Date of last trt LB and EG visit Consent W. 2014 -03 -14 Follow up? End of study, consent withdraw

How it looks in the data (Option 1) SE ROW ETCD 1 SCR 2 TRT 3 FU SESTDTC 2014 -01 -01 SEENDTC 2014 -01 -10 2014 -02 -01 2014 -03 -14 SEUPDES EPOCH SCREENING TREATMENT FOLLOW UP

How it looks in the data (cont. ) LB SV DS ROW EPOCH 1 TREATMENT Cycle 2 2 2014 -02 -01 2 FOLLOW UP Cycle 3 3 2014 -03 -07 ROW EPOCH VISITNUM SVDY SVDTC 2014 -02 -01 1 TREATMENT Cycle 2 2 2 FOLLOW-UP 3 2014 -02 -21 3 ? ? ? Cycle 3 End of Treatment 99 2014 -03 -14 4 FOLLOW-UP End of Study 999 2014 -03 -14 ROW DSCAT PROTOCOL MILESTONE DISPOSITION EVENT 1 2 3 DSSCAT DSDECODE RANDOMIZATI ON END OF TREATMENT AE END OF CONCENT STUDY WITHDRAWL LBDTC EPOCH Date, where decision to stop treatment and study was made (last subject visit) DSSTDTC SCREENING 2013 -11 -11 ? ? ? 2014 -02 -01 FOLLOW-UP 2014 -03 -14 Date of last medication administration

Question to team How to populate EPOCH for „End of Treatment“ (SV obs 3, and DS obs 2) for that case: 1. „FOLLOW UP“ in SV and DS (discrepancy with protocol schedule and SDTM IG as end of treament closed to treatment epoch)? 2. „TREATMENT“ in SV and DS (discrepancy by date and sorting order for SV, discrepancy to SE)? 3. „FOLLOW UP“ in SV and „TREATMENT“ in DS (agreement with SE based on date, but different EPOCH description for same event)? 4. Leave blank?

Element level: Subject time axis No FU information collected in Original EDC DB Last treatment 2014 -02 -01 Screening Treatment Randomization Treatment start LB and EG visit Consent W. 2014 -03 -14 Unplanned SE, Treatment delay? End of treatment, Date of last trt End of study, consent withdraw

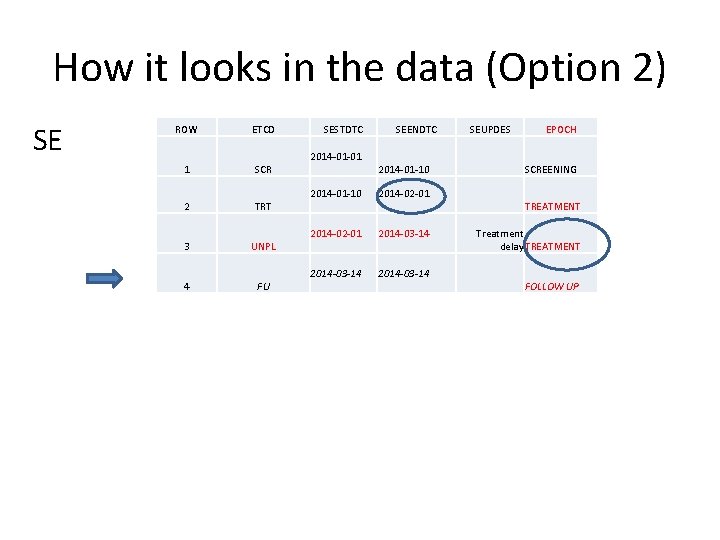
How it looks in the data (Option 2) SE ROW ETCD 1 SCR 2 TRT 3 UNPL 4 FU SESTDTC 2014 -01 -01 SEENDTC 2014 -01 -10 2014 -02 -01 2014 -03 -14 SEUPDES EPOCH SCREENING TREATMENT Treatment delay TREATMENT FOLLOW UP

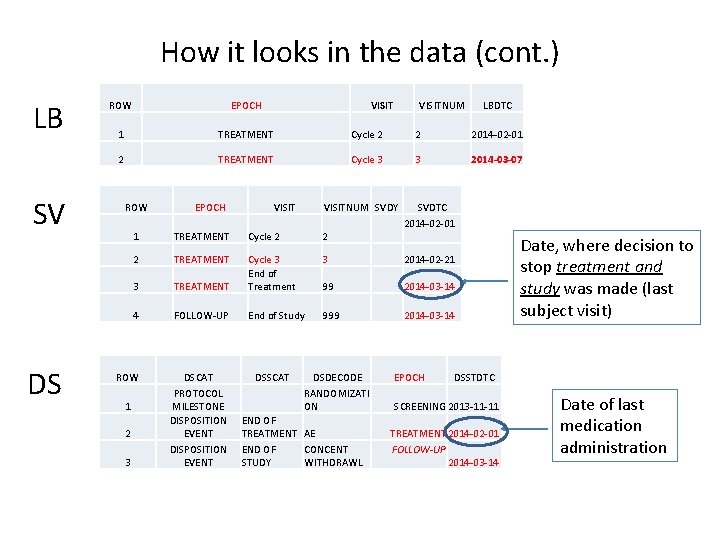
How it looks in the data (cont. ) LB SV DS ROW EPOCH 1 TREATMENT Cycle 2 2 2014 -02 -01 2 TREATMENT Cycle 3 3 2014 -03 -07 ROW EPOCH VISITNUM SVDY SVDTC 2014 -02 -01 1 TREATMENT Cycle 2 2 2 TREATMENT 3 2014 -02 -21 3 TREATMENT Cycle 3 End of Treatment 99 2014 -03 -14 4 FOLLOW-UP End of Study 999 2014 -03 -14 ROW DSCAT PROTOCOL MILESTONE DISPOSITION EVENT 1 2 3 DSSCAT DSDECODE RANDOMIZATI ON END OF TREATMENT AE END OF CONCENT STUDY WITHDRAWL LBDTC EPOCH Date, where decision to stop treatment and study was made (last subject visit) DSSTDTC SCREENING 2013 -11 -11 TREATMENT 2014 -02 -01 FOLLOW-UP 2014 -03 -14 Date of last medication administration

Questions to team • Can we consider Treatment delay as Unplanned Element if it is acceptable according to Protocol • Do we need to define „FU“ Element /Epoch for this case in SE and other domains?

Questions to FDA • Is variable EPOCH expected in SV and DS domains as well? • Is discrepancy between scheduled visit number and EPOCH acceptable if described in RG? (considering option 1 as for other subjects Cycle 3 will correspond to „TREATMENT“ EPOCH) • Would it be acceptable to have EPOCH blank with description in Reviewer Guide?
 Epoch foot treatment
Epoch foot treatment After me after me after me
After me after me after me If any man come after me
If any man come after me Counting rule for multiple-step experiment
Counting rule for multiple-step experiment Worksheet: oxidation numbers
Worksheet: oxidation numbers The process of assigning tasks to workstations
The process of assigning tasks to workstations Assigning homework and providing practice
Assigning homework and providing practice When assigning hcpcs level ii codes, _____.
When assigning hcpcs level ii codes, _____. The complement of event a
The complement of event a 5 urban hearths
5 urban hearths Smabsa
Smabsa Tribal epoch
Tribal epoch Epoch 1000
Epoch 1000 Time
Time Concentric zone model
Concentric zone model Sail wagon epoch
Sail wagon epoch Epoch 1000
Epoch 1000