Typing methods for bacteria May 2007 Laboratory Training

Typing methods for bacteria May 2007 Laboratory Training for Field Epidemiologists

Learning objectives At the end of the presentation, participants should: • Identify situations when typing is relevant • Know different methods of typing • Understand problems that arise when using typing methods Laboratory Training for Field Epidemiologists
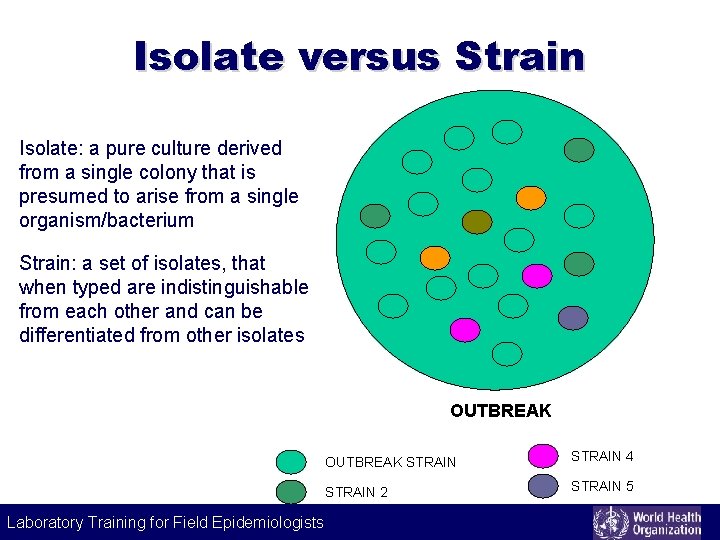
Isolate versus Strain Isolate: a pure culture derived from a single colony that is presumed to arise from a single organism/bacterium Strain: a set of isolates, that when typed are indistinguishable from each other and can be differentiated from other isolates OUTBREAK STRAIN 4 STRAIN 2 STRAIN 5 STRAIN Laboratory Training for Field Epidemiologists Laboratory Training for 3 Epidemiologists STRAIN 6

A simple question ? Are these isolates the same or different? Through a typing method we are looking for: • Epidemiologically linked isolates that represent the clonal expansion of a single precursor • Clonal isolates are the same type and unrelated isolates have a different type Laboratory Training for Field Epidemiologists Laboratory Training for Epidemiologists
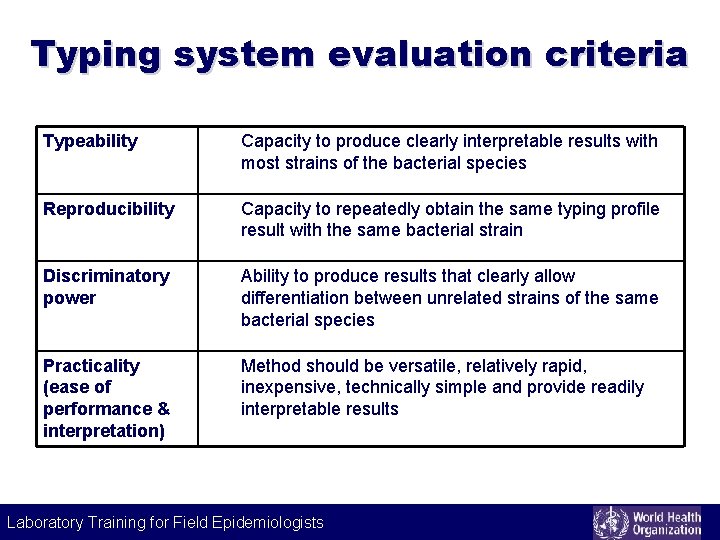
Typing system evaluation criteria Typeability Capacity to produce clearly interpretable results with most strains of the bacterial species Reproducibility Capacity to repeatedly obtain the same typing profile result with the same bacterial strain Discriminatory power Ability to produce results that clearly allow differentiation between unrelated strains of the same bacterial species Practicality (ease of performance & interpretation) Method should be versatile, relatively rapid, inexpensive, technically simple and provide readily interpretable results Laboratory Training for Field Epidemiologists Laboratory Training for Epidemiologists
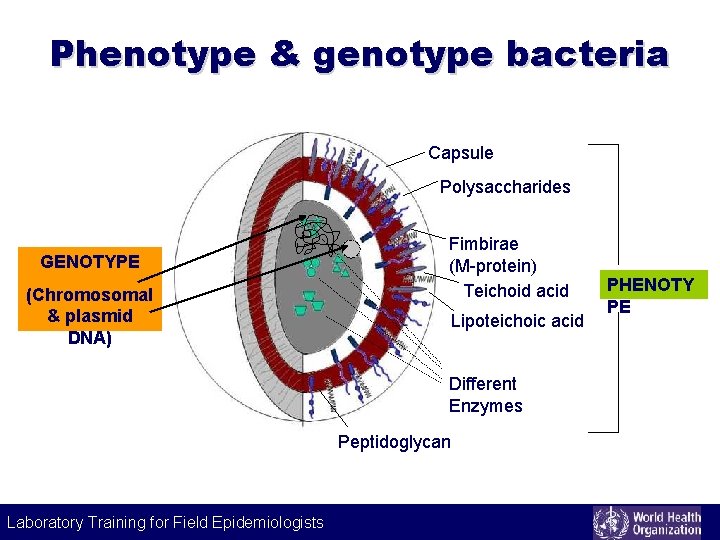
Phenotype & genotype bacteria Capsule Polysaccharides GENOTYPE (Chromosomal & plasmid DNA) Fimbirae (M-protein) Teichoid acid Lipoteichoic acid Different Enzymes Peptidoglycan Laboratory Training for Field Epidemiologists Laboratory Training for Epidemiologists PHENOTY PE
Typing methods Phenotypic • Rely on expression of phenotypic characteristics (genetically coded) – Antibiotic resistance, antigens etc. Genotypic • Analysis of the genetic material – DNA, RNA Laboratory Training for Field Epidemiologists Laboratorz Training for Epidemiologists
Phenotypic techniques Serotyping Phage typing Antimicrobial resistance monitoring Multilocus enzyme electrophoresis (MLEE) Other: • Protein profiling – SDS PAGE, immunoblotting • Based on nutritional requirement e. g. auxotyping • Biotyping • Bacteriocin typing Laboratory Training for Field Epidemiologists Laboratory Training for Epidemiologists
Phenotypic techniques Serotyping • Antigenetic determinants expressed on the cell surface • Still widely used for Salmonella, Shigella, Neiseria, E. coli, V cholerae • Slide/ tube agglutination • LIMITATION: Requires extensive stock of absorbed/monoclonal sera (e. g. >2200 antisera required for definitive Salmonella typing) Phage typing • Viruses that infect and destroy bacterial cells –Bacteriophage • The resistance or susceptibility of strains is used for differentiation • LIMITATION: Technically demanding, time consuming, typeability is an issue Laboratory Training for Field Epidemiologists
Phenotypic techniques Antibiotic susceptibility testing • Based on susceptibility of bacterial isolates to a panel of antimicrobial agents • Routinely performed on clinical isolates • A reasonable preliminary indicator to initiate epidemiological action LIMITATIONS: – Antibiotic resistance under extraordinary selective pressure – Multiple mechanisms for a strain to become abruptly resistant Laboratory Training for Field Epidemiologists Laboratory Training for Epidemiologists
Phenotypic Techniques Phenotypic characteristics can vary in different conditions • Antibiotic resistance can be expressed under antibiotic pressure Methods are not very discriminatory Laboratory Training for Field Epidemiologists Laboratory Training for Epidemiologists
MLEE Characterizes the cellular proteins by electrophoretically separating them in a gel matrix Exposing the gel to chromogenic substrates (that react with the enzymes) Limitation: Complexity of interpretation Laboratory Training for Field Epidemiologists
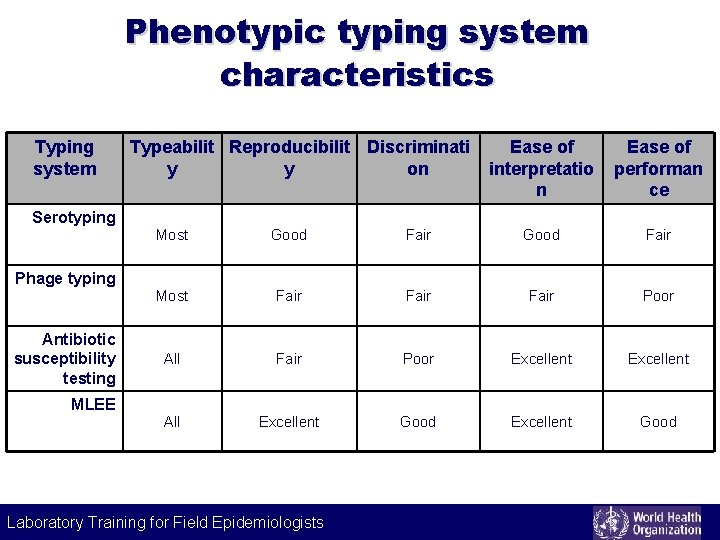
Phenotypic typing system characteristics Typing system Serotyping Typeabilit Reproducibilit Discriminati y y on Ease of interpretatio n Ease of performan ce Most Good Fair Most Fair Poor All Fair Poor Excellent All Excellent Good Phage typing Antibiotic susceptibility testing MLEE Laboratory Training for Field Epidemiologists Laboratory Training for Epidemiologists
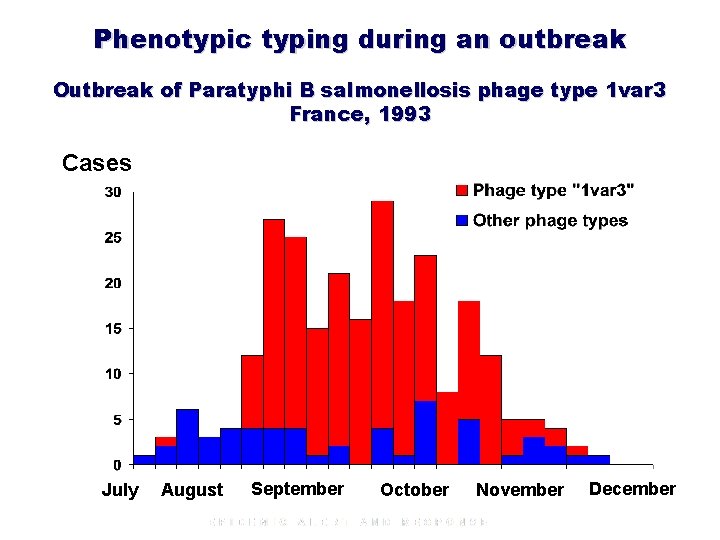
Phenotypic typing during an outbreak Outbreak of Paratyphi B salmonellosis phage type 1 var 3 France, 1993 Cases July August September October November December
DNA molecule Source: Wikipedia, created by Michael Ströck Laboratory Training for Field Epidemiologists Laboratory Training for Epidemiologists
Genotypic methods Plasmid profiling Restriction enzyme analysis (REA) Restriction fragment length polymorphism (RFLP) Ribotyping Pulse Field Gel Electrophoresis (PFGE) Random Amplified Polymorphic DNA (RAPD) Nucleic acid sequencing Laboratory Training for Field Epidemiologists Laboratory Training for Epidemiologists
The principle Without amplification • Cutting the DNA in pieces • Visualizing the pieces With amplification • Amplifying (using PCR) parts of the DNA • Visualizing the pieces Laboratory Training for Field Epidemiologists Laboratory Training for Epidemiologists
Genotypic typing methods Methods without prior amplification • Isolation of the pathogen • Extraction of the DNA • Cutting the DNA with Enzymes (restriction endonuclease enzymatically cuts/ “digests” DNA at a specific/ “restricted” nucleotide recognition sequence) • Separation of the pieces by size using an electric field (Gel. Electrophoresis) • Visualization Laboratory Training for Field Epidemiologists Laboratory Training for Epidemiologists
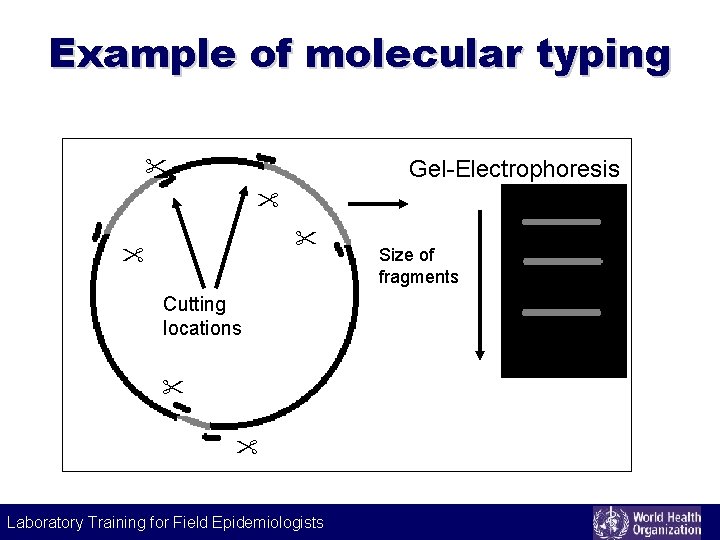
Example of molecular typing Gel-Electrophoresis Size of fragments Cutting locations Laboratory Training for Field Epidemiologists Laboratory Training for Epidemiologists
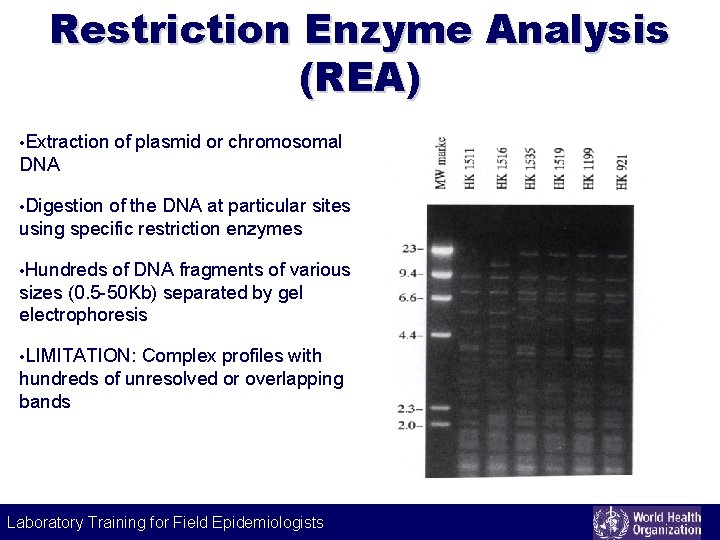
Restriction Enzyme Analysis (REA) • Extraction of plasmid or chromosomal DNA • Digestion of the DNA at particular sites using specific restriction enzymes • Hundreds of DNA fragments of various sizes (0. 5 -50 Kb) separated by gel electrophoresis • LIMITATION: Complex profiles with hundreds of unresolved or overlapping bands Laboratory Training for Field Epidemiologists Laboratory Training for Epidemiologists
Southern blot analysis of RFLP & ribotyping Better analysis of restriction enzyme patterns • Specific parts of the pieces are detected by pieces of DNA as a probe - Southern Blot • Variation in number & size of fragments detected by the markers are referred to as restriction fragment length polymorphism (RFLP) • Ribotyping: when probes mark ribosomal operons LIMITATIONS: technically complex, organisms with single copy of ribosomal operons Laboratory Training for Field Epidemiologists Laboratory Training for Epidemiologists
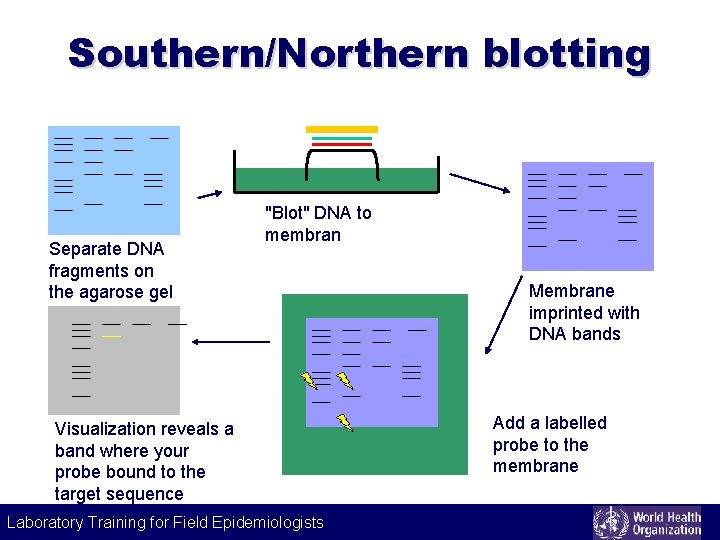
Southern/Northern blotting Separate DNA fragments on the agarose gel Visualization reveals a band where your probe bound to the target sequence "Blot" DNA to membran Membrane imprinted with DNA bands Add a labelled probe to the membrane Laboratory Training for Field Epidemiologists Laboratory Training for Epidemiologists
Visualization with a marker _I I I_ __ _I I I_ __ I_ _I I __ _I I I_ _I I I_ Adapted from: http: //lifesciences. asu. edu/resources/mamajis/southern. html Laboratory Training for Field Epidemiologists Laboratory Training for Epidemiologists
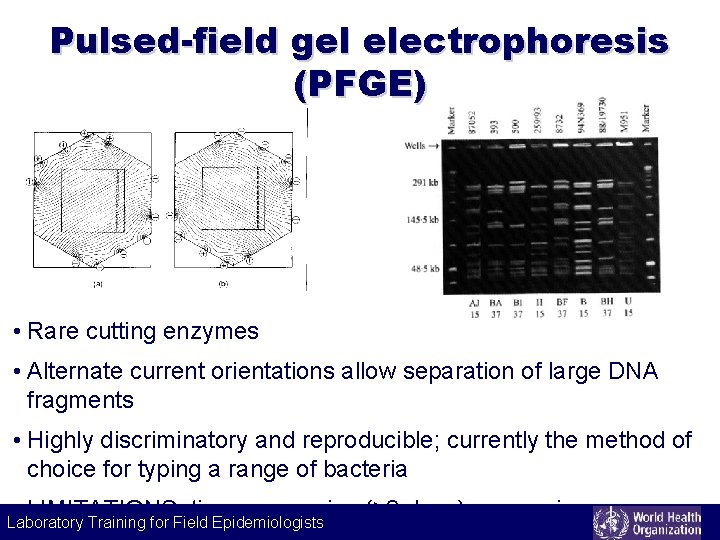
Pulsed-field gel electrophoresis (PFGE) • Rare cutting enzymes • Alternate current orientations allow separation of large DNA fragments • Highly discriminatory and reproducible; currently the method of choice for typing a range of bacteria LIMITATIONS: time consuming (≥ 2 days), expensive, Laboratory Training for Field Epidemiologists Laboratory Training for Epidemiologists
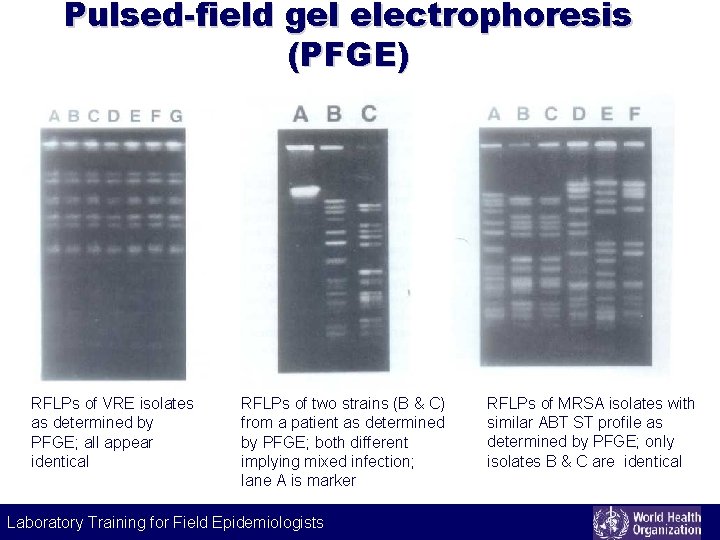
Pulsed-field gel electrophoresis (PFGE) RFLPs of VRE isolates as determined by PFGE; all appear identical RFLPs of two strains (B & C) from a patient as determined by PFGE; both different implying mixed infection; lane A is marker RFLPs of MRSA isolates with similar ABT ST profile as determined by PFGE; only isolates B & C are identical Laboratory Training for Field Epidemiologists Laboratory Training for Epidemiologists
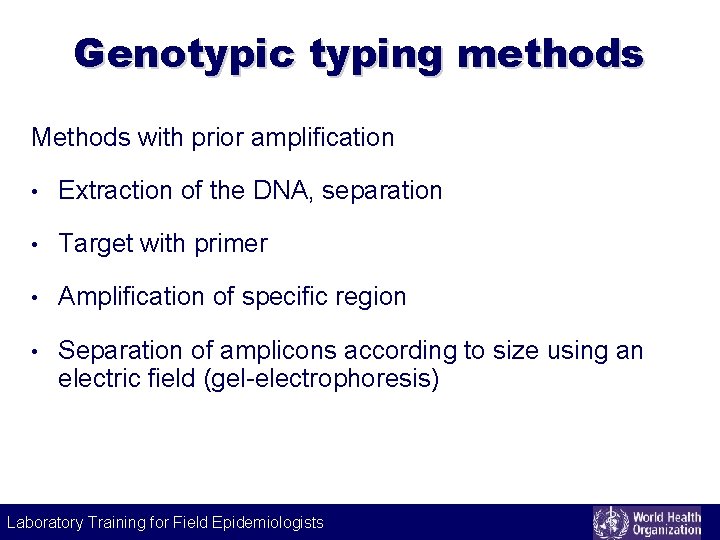
Genotypic typing methods Methods with prior amplification • Extraction of the DNA, separation • Target with primer • Amplification of specific region • Separation of amplicons according to size using an electric field (gel-electrophoresis) Laboratory Training for Field Epidemiologists Laboratory Training for Epidemiologists
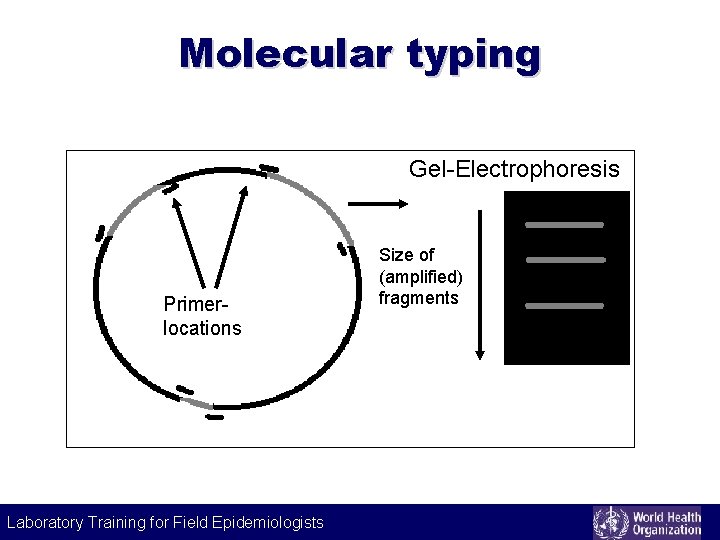
Molecular typing Gel-Electrophoresis Primerlocations Size of (amplified) fragments Laboratory Training for Field Epidemiologists Laboratory Training for Epidemiologists

Random Amplification of Polymorphic DNA (RAPD ) Uses short primers that find a lot of targets Different size amplicons Products separated by electrophoresis LIMTATIONS: • Identification of suitable primers • Difficult to interpret differences in the intensity of bands • Inefficient reactions • Amplification of cryptic genetic material (prophages, bacteriophages) Laboratory Training for Field Epidemiologists Laboratory Training for Epidemiologists
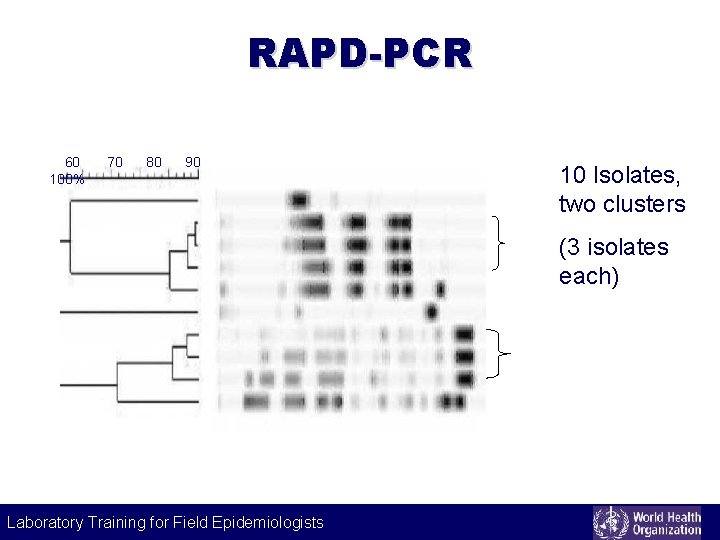
RAPD-PCR 60 100% 70 80 90 10 Isolates, two clusters (3 isolates each) Laboratory Training for Field Epidemiologists Laboratory Training for Epidemiologists
Nucleic acid sequencing Enumeration of individual nucleotide base pairs Provides highly reliable and objective data suitable for subsequent quantitative analysis Necessary for virus typing LIMITATIONS: • Locus with sufficient sequence variability • Sequencing of a single locus may not be reliable result • Prohibitively expensive for most settings Laboratory Training for Field Epidemiologists Laboratory Training for Epidemiologists
Multi Locus sequence typing (MLST) Targets different DNA pieces and sequences them Compares results with data banks Pro: highly comparable Con: expensive equipment Laboratory Training for Epidemiologists
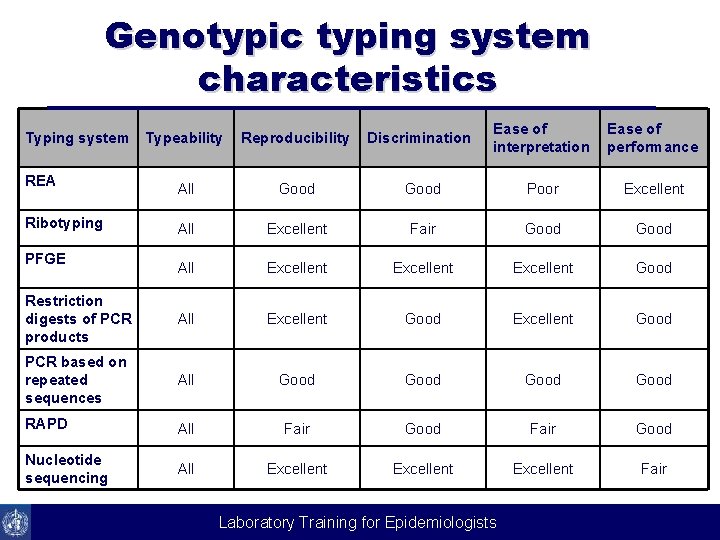
Genotypic typing system characteristics Typeability Reproducibility Discrimination Ease of interpretation Ease of performance All Good Poor Excellent All Excellent Fair Good All Excellent Good Restriction digests of PCR products All Excellent Good PCR based on repeated sequences All Good RAPD All Fair Good Nucleotide sequencing All Excellent Fair Typing system REA Ribotyping PFGE Laboratory Training for Epidemiologists
Limitations of typing methods • Discriminatory function • Type of material and pathogen • Reproducibility , cost, technique, etc. • No “gold standard” RESULTS OF A TYPING SYSTEM SHOULD BE CONSIDERED RELATIVE TO THE AVAILABLE EPIDEMIOLOGICAL DATA OR TO THE RESULTS OF OTHER SYSTEMS • The technique used needs to be adapted to the question Laboratory Training for Epidemiologists
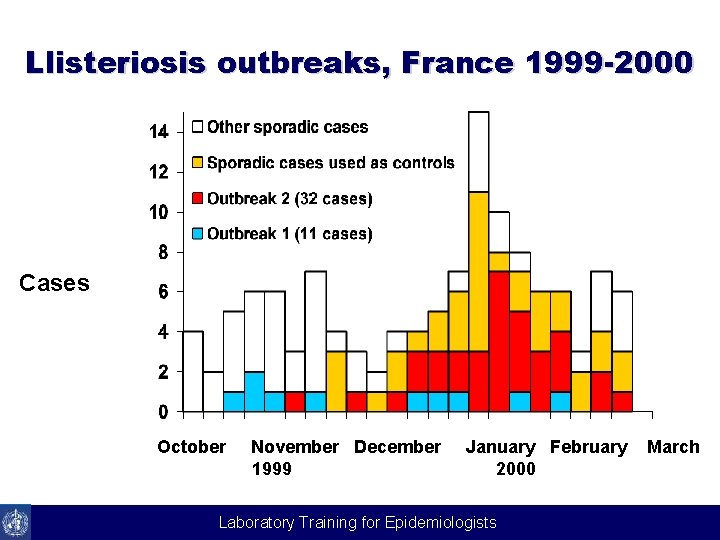
Llisteriosis outbreaks, France 1999 -2000 Cases October November December 1999 January February 2000 Laboratory Training for Epidemiologists March
Interpretation of strain typing data Several factors affect interpretation: Natural biologic variation • Epidemiologically related isolates of the same strain demonstrate minor typing differences due to phenotypic variations or actual genotypic alterations, when collected and examined over an extended interval Technical variations • Limited reproducibility and discriminatory power Laboratory Training for Epidemiologists
Interpretation of typing results Genetic relatedness assessed with clinical and epidemiologic relatedness Restrict analysis to discrete set of isolates (≤ 30) Identify “index isolate” as starting point for analysis that is defined on the basis of: • Epidemiological data (first case in an outbreak) • Clinical data (initial isolate from patient with multiple infections) • Strain typing data (most common strain type in the set) Laboratory Training for Epidemiologists
Interpretation of typing results Multiple isolates representing a single type are most appropriately designated “indistinguishable” No typing method confirms that entire genomes of two organisms are identical Indistinguishable vs. closely related vs. possibly related →Final assessment lies with integration of molecular and epidemiological analyses Laboratory Training for Epidemiologists
Problems with result interpretation Types versus subtypes Isolates assigned as different types if differ in some specified manner (2 or more band shift in S blot) Isolates that differ but not sufficiently to be designated as distinct types are designated as subtypes of similar types Restriction fragments of different sizes may represent same chromosomal DNA Insertion or deletion of extra-chromosomal DNA such as bacteriophage DNA fragment data are not suitable for quantitating genetic relatedness among different isolates Laboratory Training for Epidemiologists
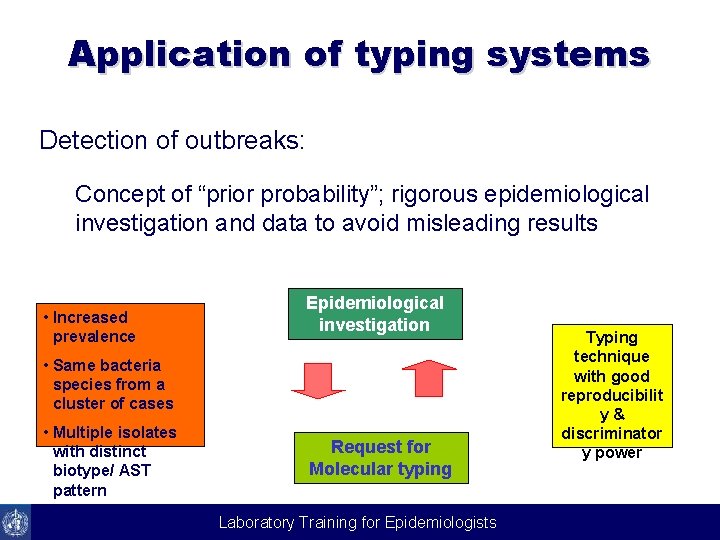
Application of typing systems Detection of outbreaks: Concept of “prior probability”; rigorous epidemiological investigation and data to avoid misleading results • Increased prevalence Epidemiological investigation • Same bacteria species from a cluster of cases • Multiple isolates with distinct biotype/ AST pattern Request for Molecular typing Laboratory Training for Epidemiologists Typing technique with good reproducibilit y& discriminator y power
Applications of typing systems Distinguish relapse from re-infection Identify types associated with increased transmission & virulence Emergence of new types ; implications on control measures Clonality of acute infection: • Infection vs. colonization vs. sample contamination • Pseudo-outbreaks related to: – Clinician or clinical entity – Laboratory – Case finding – Chance clustering Laboratory Training for Epidemiologists
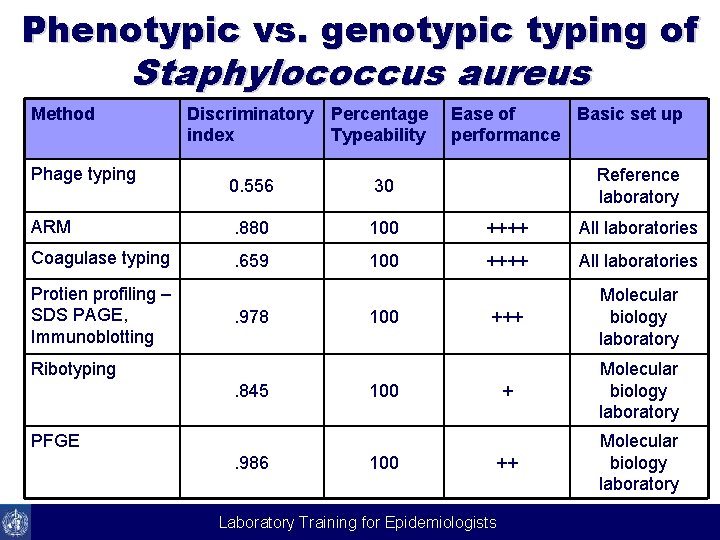
Phenotypic vs. genotypic typing of Staphylococcus aureus Method Discriminatory index Percentage Typeability 0. 556 30 ARM . 880 100 ++++ All laboratories Coagulase typing . 659 100 ++++ All laboratories +++ Molecular biology laboratory Phage typing Protien profiling – SDS PAGE, Immunoblotting . 978 100 Ease of Basic set up performance Reference laboratory Ribotyping. 845 100 PFGE. 986 100 Laboratory Training for Epidemiologists

To summarize Typing data are most appropriately evaluated in the context of a hypothesis and questions thoughtfully developed by the clinician or the epidemiologist. They should augment rather that replace those analyses Typing is performed independently by the laboratory to avoid any bias but the results are considered collaboratively Laboratory Training for Epidemiologists

Typing Developed by the Department of Epidemic and Pandemic Alert and Response of the World Health Organization with assistance from: European Program for Intervention Epidemiology Training Canadian Field Epidemiology Program Thailand Ministry of Health Institut Pasteur Laboratory Training for Field Epidemiologists
- Slides: 43