Types of solution stoichiometry problems Dilutions Gravimetric analysis
Types of solution stoichiometry problems • Dilutions • Gravimetric analysis • Titrations 12/26/2021 1

Gravimetric Analysis Determining % (by mass) of an element in an unknown ionic compound 1. Dissolve unknown substance in water 2. React unknown with known substance to form a precipitate 3. Filter and dry precipitate 4. Weigh precipitate 5. Use chemical formula and mass of precipitate to determine amount of unknown ion 12/26/2021 2
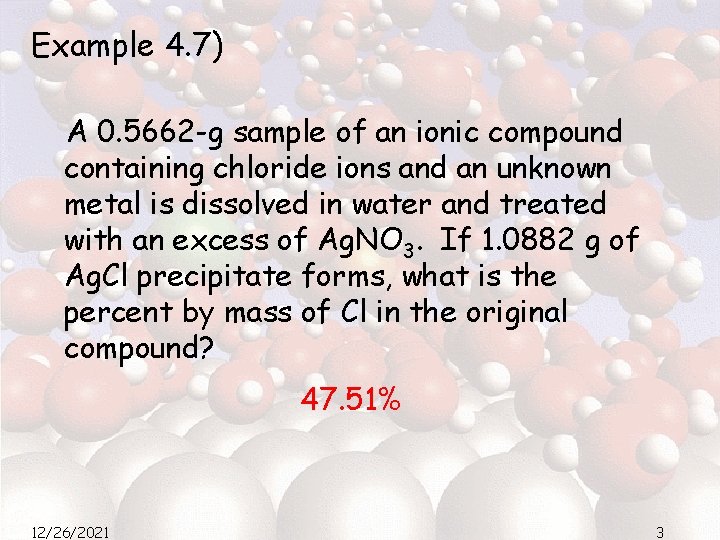
Example 4. 7) A 0. 5662 -g sample of an ionic compound containing chloride ions and an unknown metal is dissolved in water and treated with an excess of Ag. NO 3. If 1. 0882 g of Ag. Cl precipitate forms, what is the percent by mass of Cl in the original compound? 47. 51% 12/26/2021 3

4. 71) If 30. 0 m. L of 0. 150 M Ca. Cl 2 is added to 15. 0 m. L of 0. 100 M Ag. NO 3, what is the mass in grams of Ag. Cl precipitate? 0. 215 g Ag. Cl …now try 4. 73! 12/26/2021 4

Titrations In a titration a solution of accurately known concentration is added gradually added to another solution of unknown concentration until the chemical reaction between the two solutions is complete. Equivalence point – the point at which the reaction is complete Indicator – substance that changes color at (or near) the equivalence point Slowly add base to unknown acid UNTIL the indicator changes color 12/26/2021 5
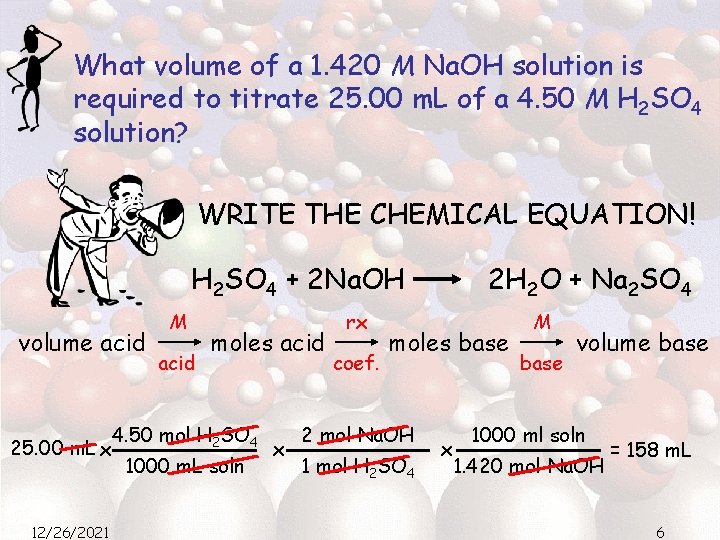
What volume of a 1. 420 M Na. OH solution is required to titrate 25. 00 m. L of a 4. 50 M H 2 SO 4 solution? WRITE THE CHEMICAL EQUATION! H 2 SO 4 + 2 Na. OH volume acid 25. 00 m. L x 12/26/2021 M acid moles acid 4. 50 mol H 2 SO 4 1000 m. L soln x rx coef. 2 H 2 O + Na 2 SO 4 moles base 2 mol Na. OH 1 mol H 2 SO 4 x M base volume base 1000 ml soln 1. 420 mol Na. OH = 158 m. L 6

Example 4. 8) In a titration experiment, a student finds that 23. 48 m. L of a Na. OH solution are needed to neutralize 0. 5468 g of KHP. What is the concentration (in molarity, M) of the Na. OH solution? KHP = KHC 8 H 4 O 4 Molar mass of KHP= 204. 2 g 0. 1141 M …now try 4. 77! 12/26/2021 7

Gases Chapter 5: 130 -167
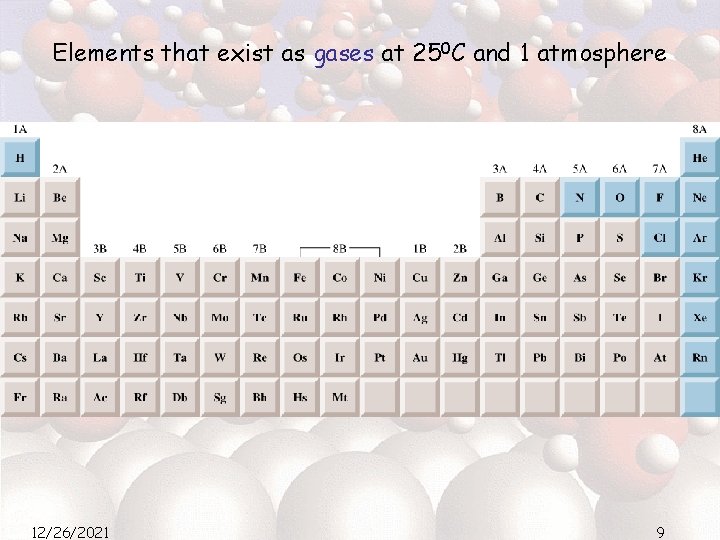
Elements that exist as gases at 250 C and 1 atmosphere 12/26/2021 9
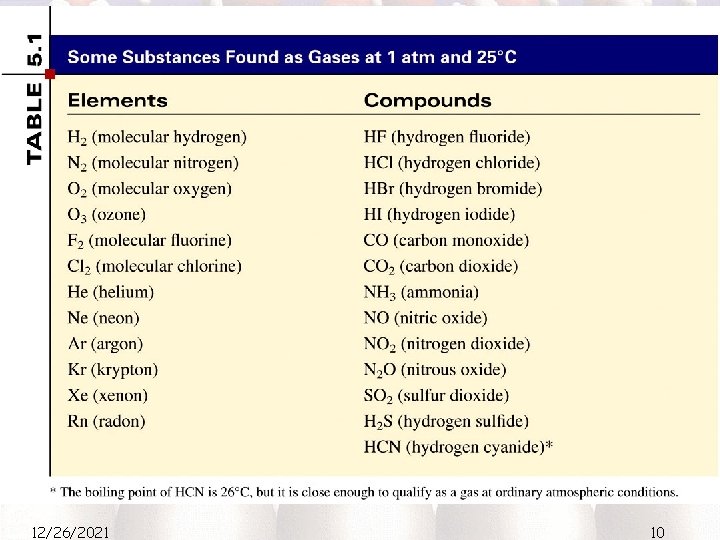
12/26/2021 10
Physical Characteristics of Gases • Gases assume the volume and shape of their containers. • Gases are the most compressible state of matter. • Gases will mix evenly and completely when confined to the same container. • Gases have much lower densities than liquids and solids. 12/26/2021 11
- Slides: 11