Types of Solids There are three main types









- Slides: 23

Types of Solids • There are three main types of solid: – Crystalline solids – Amorphous solids – Polymers

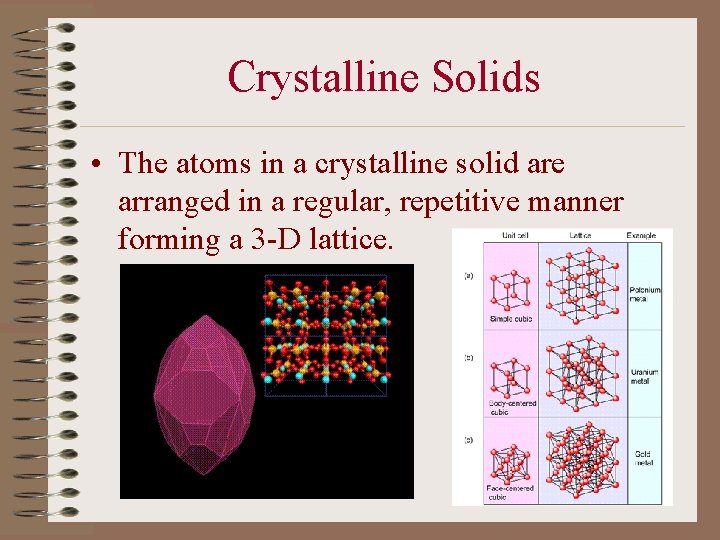
Crystalline Solids • The atoms in a crystalline solid are arranged in a regular, repetitive manner forming a 3 -D lattice.

Amorphous Solids • Solids which have their atoms arranged in a completely irregular structure are called amorphous (without shape) solids. • e. g. Glass

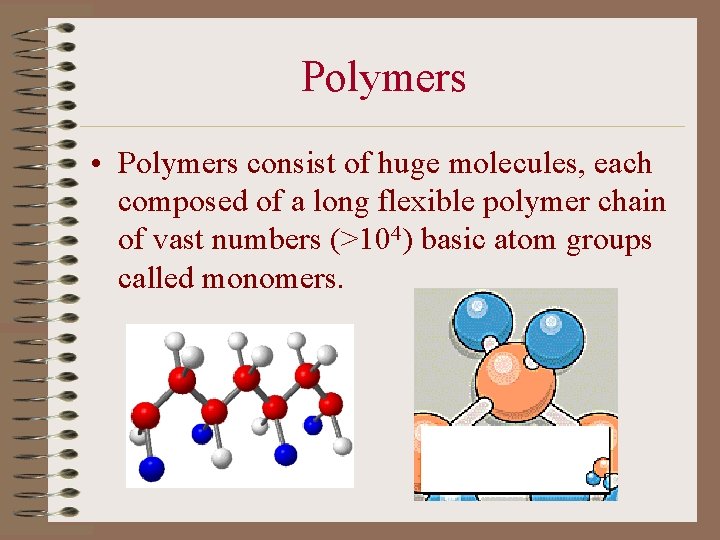
Polymers • Polymers consist of huge molecules, each composed of a long flexible polymer chain of vast numbers (>104) basic atom groups called monomers.

Mechanical Properties of Solids (1) • Strength – The force required to break a given material is a measure of its strength. – The breaking force depends on • the shape of the solid, • the size of the solid, • the type of the material.

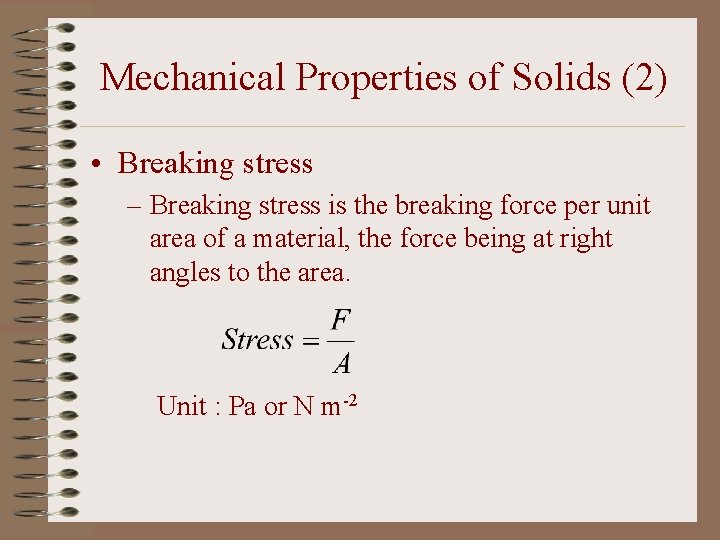
Mechanical Properties of Solids (2) • Breaking stress – Breaking stress is the breaking force per unit area of a material, the force being at right angles to the area. Unit : Pa or N m-2

Mechanical Properties of Solids (3) • Stiffness – Stiffness is a measure of the difficulty of changing the shape of an object. – The stiffness is measured by the Young’s modulus of the material. • Brittleness – Brittle materials are very stiff but will snap if too much force is applied.

Mechanical Properties of Solids (4) • Ductility – Materials which show a large amount of plastic deformation under stress are said to be ductile. • Plastic behaviour – Plastic behaviour occurs when a material is deformed beyond its elastic limit. – In plastic deformation, bonds between atoms are broken one at a time.

Mechanical Properties of Solids (5) • Hardness – Hardness is a measure of the difficulty of scratching a material. • Creep – Creep is the continuous deformation that occurs from prolonged static stress. – Creep occurs when a material, acted on by constant forces, changes its shape even though the forces on it remain constant.

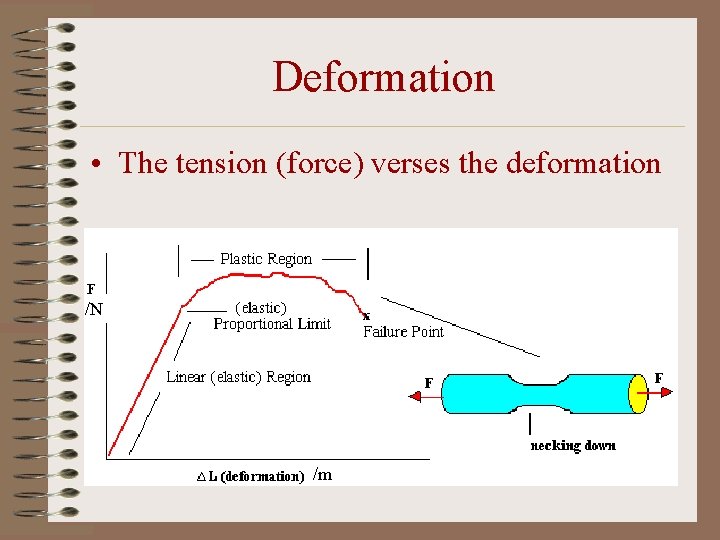
Deformation • The tension (force) verses the deformation /N /m

Elasticity • The elasticity of a body is its ability to return to its original form after the distorting forces have been removed.

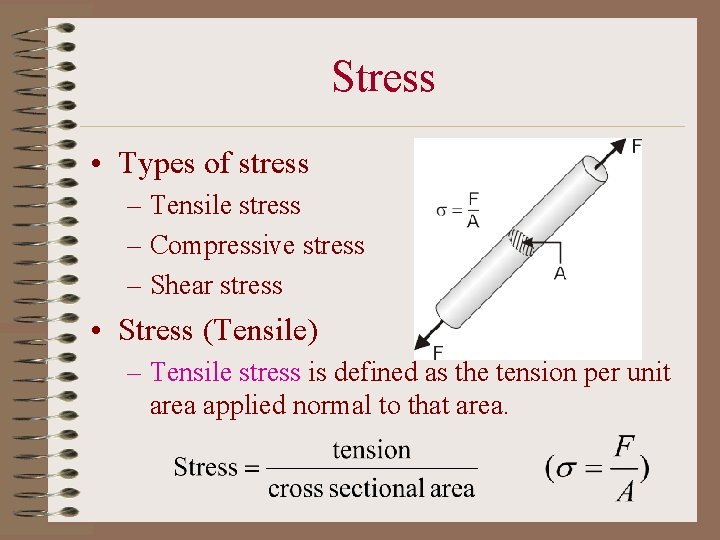
Stress • Types of stress – Tensile stress – Compressive stress – Shear stress • Stress (Tensile) – Tensile stress is defined as the tension per unit area applied normal to that area.

Strain • Strain is a measure of the extent of deformation of an object. • Strain is defined as the extension of an object per unit length. lo l

Hooke’s Law • When stress is applied to a material, strain is produced in the material. • Strain stress provided the limit of proportionality is not exceeded. where E is called the Young modulus.

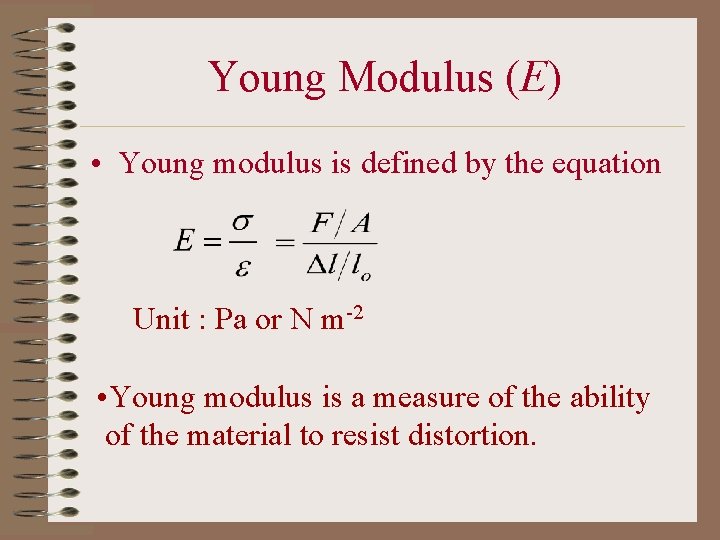
Young Modulus (E) • Young modulus is defined by the equation Unit : Pa or N m-2 • Young modulus is a measure of the ability of the material to resist distortion.

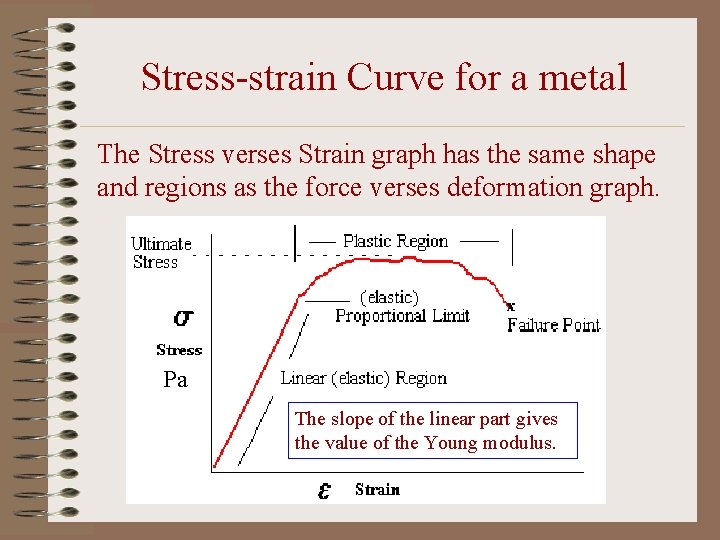
Stress-strain Curve for a metal The Stress verses Strain graph has the same shape and regions as the force verses deformation graph. Pa The slope of the linear part gives the value of the Young modulus.

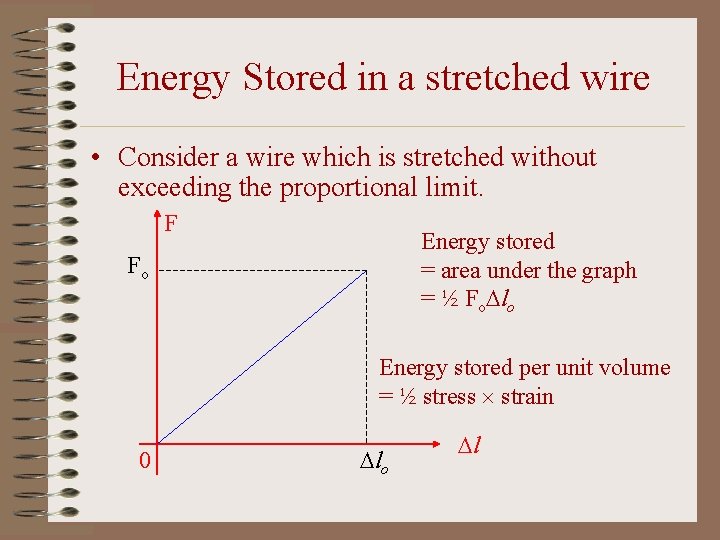
Energy Stored in a stretched wire • Consider a wire which is stretched without exceeding the proportional limit. F Energy stored = area under the graph = ½ Fo lo Fo Energy stored per unit volume = ½ stress strain 0 lo l

A Model for a solid • A model is something which allows you to describe and explain some phenomenon. • Mathematical model – Hooke’s law is a mathematical model. It is a successful model because of its universality and simplicity. • Analogical model – The behaviour of solids can be described by a model which assumes that matter is made of atoms with the properties of sticky tennis balls.

Intermolecular Forces • The intermolecular forces arises from two main causes: – The potential energy of the molecules, which is due to the electromagnetic interactions with surrounding molecules. – The thermal energy of the molecules, which is the KE of the molecules and it depends on the temperature.

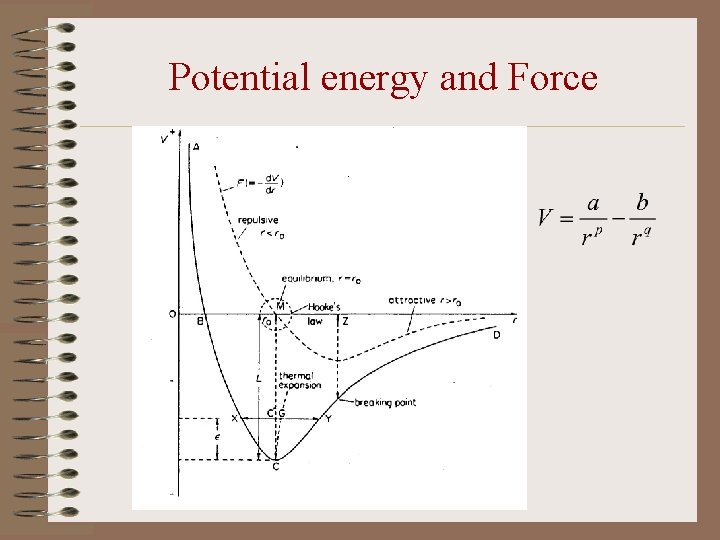
Potential energy and Force

Properties of Solids from Molecular Theory (1) • Equilibrium spacing of molecules (r = ro) – The potential energy is minimum. – The repulsive force and the attractive force balance. – The molecules oscillate about their equilibrium position. • Elasticity – Near the equilibrium position, r F. – Intermolecular force constant, k = - d. F/dr. – Young’s modulus = k/ro

Properties of Solids from Molecular Theory (2) • Breaking strain – Beyond a separation, r = OZ, the restoring force decreases with increasing separation. – OZ is the separation between molecules at the breaking point of the solid. – Breaking strain = MZ/ro. • Vaporization – When the energy equals CM (latent heat), the molecules have little interaction and form a gas.

Properties of Solids from Molecular Theory (3) • Thermal Expansion – At a higher temperature, the mean position of the oscillation shifts to right due to the asymmetry of the curve. – This corresponds to a greater separation than ro. Thus the solid expands.