Types of Reactions There are five types of










- Slides: 16

Types of Reactions • There are five types of chemical reactions we will talk about: 1. 2. 3. 4. 5. • Synthesis reactions Decomposition reactions Single displacement reactions Double displacement reactions Combustion reactions You need to be able to identify the type of reaction

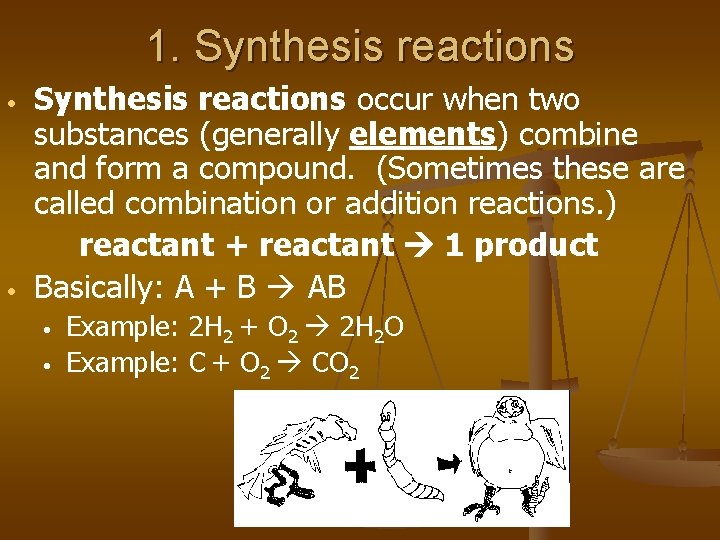
1. Synthesis reactions • • Synthesis reactions occur when two substances (generally elements) combine and form a compound. (Sometimes these are called combination or addition reactions. ) reactant + reactant 1 product Basically: A + B AB • • Example: 2 H 2 + O 2 2 H 2 O Example: C + O 2 CO 2

Synthesis Reactions • Here is another example of a synthesis reaction

2. Decomposition Reactions • • • Decomposition reactions occur when a compound breaks up into the elements or into simpler compounds 1 Reactant Product + Product In general: AB A + B Example: 2 H 2 O 2 H 2 + O 2 Example: 2 Hg. O 2 Hg + O 2

Decomposition Reactions • Another view of a decomposition reaction:

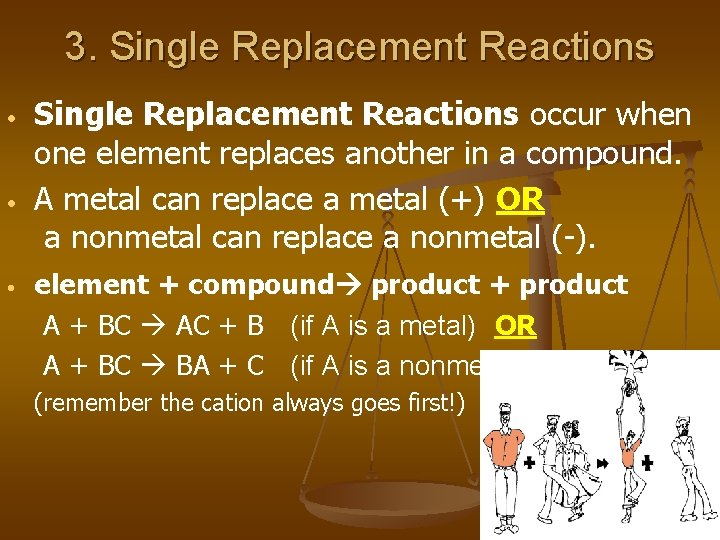
3. Single Replacement Reactions • • • Single Replacement Reactions occur when one element replaces another in a compound. A metal can replace a metal (+) OR a nonmetal can replace a nonmetal (-). element + compound product + product A + BC AC + B (if A is a metal) OR A + BC BA + C (if A is a nonmetal) (remember the cation always goes first!)

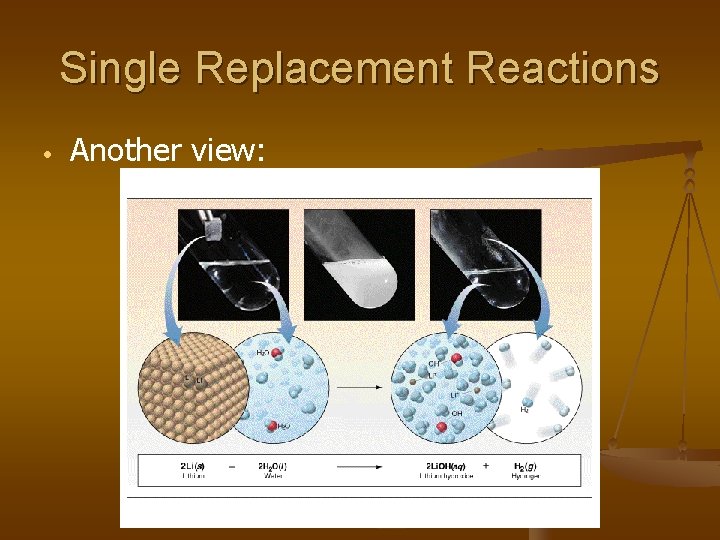
Single Replacement Reactions • Another view:

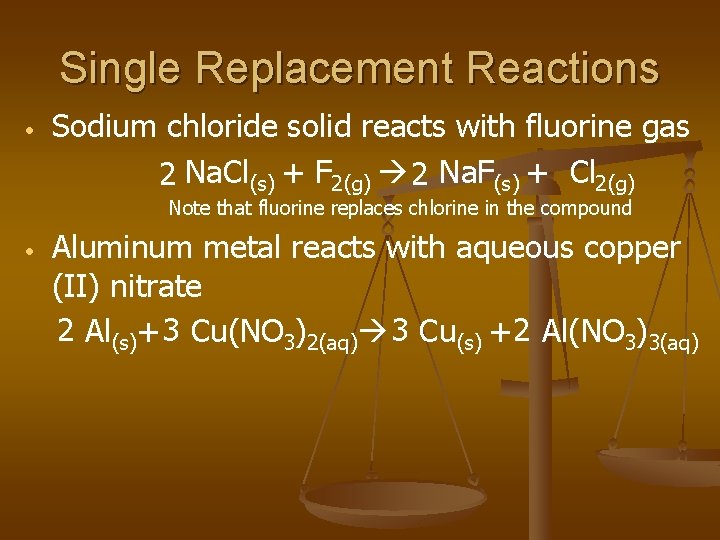
Single Replacement Reactions • Sodium chloride solid reacts with fluorine gas 2 Na. Cl(s) + F 2(g) 2 Na. F(s) + Cl 2(g) Note that fluorine replaces chlorine in the compound • Aluminum metal reacts with aqueous copper (II) nitrate 2 Al(s)+ 3 Cu(NO 3)2(aq) 3 Cu(s) + 2 Al(NO 3)3(aq)

4. Double Replacement Reactions • • • Double Replacement Reactions occur when a metal replaces a metal in a compound a nonmetal replaces a nonmetal in a compound Compound + compound product + product AB + CD AD + CB

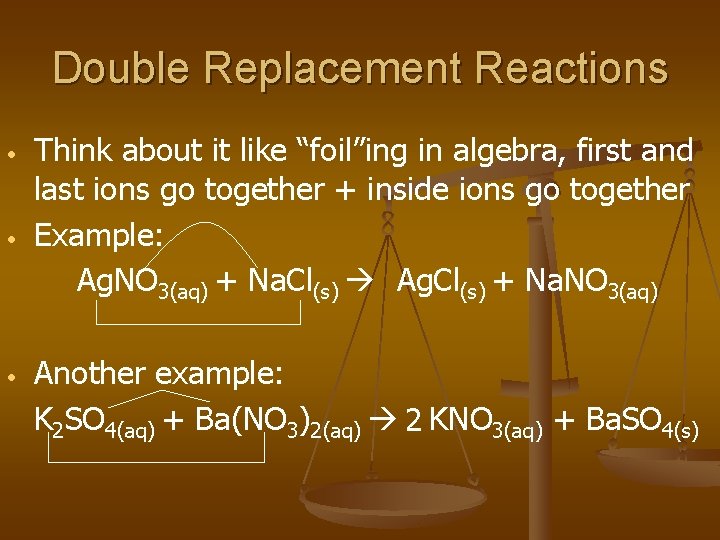
Double Replacement Reactions • • • Think about it like “foil”ing in algebra, first and last ions go together + inside ions go together Example: Ag. NO 3(aq) + Na. Cl(s) Ag. Cl(s) + Na. NO 3(aq) Another example: K 2 SO 4(aq) + Ba(NO 3)2(aq) 2 KNO 3(aq) + Ba. SO 4(s)

5. Combustion Reactions • • Combustion reactions occur when a hydrocarbon reacts with oxygen gas. This is also called burning!!! In order to burn something you need the 3 things in the “fire triangle”: 1) A Fuel (hydrocarbon) 2) Oxygen to burn it with 3) Something to ignite the reaction (spark)

Combustion Reactions • • • In general: Cx. Hy + O 2 CO 2 + H 2 O Products in combustion are ALWAYS carbon dioxide and water. (although incomplete burning does cause some byproducts like carbon monoxide) Combustion is used to heat homes and run automobiles (octane, as in gasoline, is C 8 H 18)

Combustion • Example • • C 5 H 12 + 8 O 2 5 CO 2 + 6 H 2 O Write the products and balance the following combustion reaction: • 11 H 2 O 2 C 10 H 22 +31 O 2 20 10 CO 2 +22

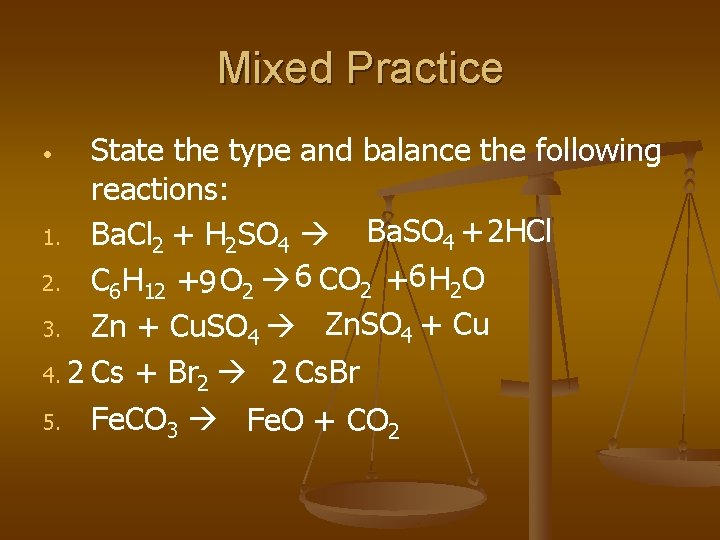
Mixed Practice State the type and balance the following reactions: Ba. SO 4 + 2 HCl 1. Ba. Cl 2 + H 2 SO 4 2. C 6 H 12 +9 O 2 6 CO 2 +6 H 2 O 3. Zn + Cu. SO 4 Zn. SO 4 + Cu 4. 2 Cs + Br 2 2 Cs. Br 5. Fe. CO 3 Fe. O + CO 2 •

Reminders to write equations n n n Remember Br. INCl. HOF (these are all two atoms together) Balance the charges using the crossing over method. (Mg 2+Cl-1)= (Mg 1 Cl 2)=(Mg. Cl 2) Identify the polyatomic Ions (ate, ite) endings ex. Phosphate = PO 43 -

n n Roman numerals tell you the charge of an atom ex. Copper (II)= Cu 2+ Don’t forget to balance out all atoms
 Insidan region jh
Insidan region jh What are the five general types of chemical reactions
What are the five general types of chemical reactions 5 types of reaction
5 types of reaction Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Redox examples
Redox examples Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers 5 types of questions in english
5 types of questions in english The sounding cataract haunted me
The sounding cataract haunted me Five of five
Five of five 5 elements and 5 senses
5 elements and 5 senses Drum and colors macbeth
Drum and colors macbeth There were five in the bed
There were five in the bed There are five flights daily from pittsburgh
There are five flights daily from pittsburgh Show that there are exactly five 3-digit cube numbers.
Show that there are exactly five 3-digit cube numbers. Types of chemical reactions redox
Types of chemical reactions redox