Types of Reactions There are FIVE main types











- Slides: 22

Types of Reactions • • There are FIVE main types of chemical reactions You need to be able to identify the type of reaction, predict the product(s), and balance the equation

Steps to Writing Formula Equations 1. 2. 3. Identify the type of reaction Predict the product(s) using the type of reaction as a model Balance it (don’t forget to reduce coefficients!) Don’t forget about the diatomic elements! (Br. INCl. HOF) For example, Oxygen O 2 is a molecule.

Caution!!! • • • The new products of a chemical reaction are the result of breaking chemical bonds and forming new ones Therefore, the products MUST form according to the ion charges and the criss-cross method for writing formulas This means the subscripts on the products may not always match the subscripts of the reactants!

1. Synthesis reactions • • Synthesis reactions occur when two substances (generally elements) combine and form a compound. (Sometimes these are called combination or addition reactions) reactant + reactant 1 product Basically: A + B AB • • Example: 2 H 2 + O 2 2 H 2 O Example: C + O 2 CO 2

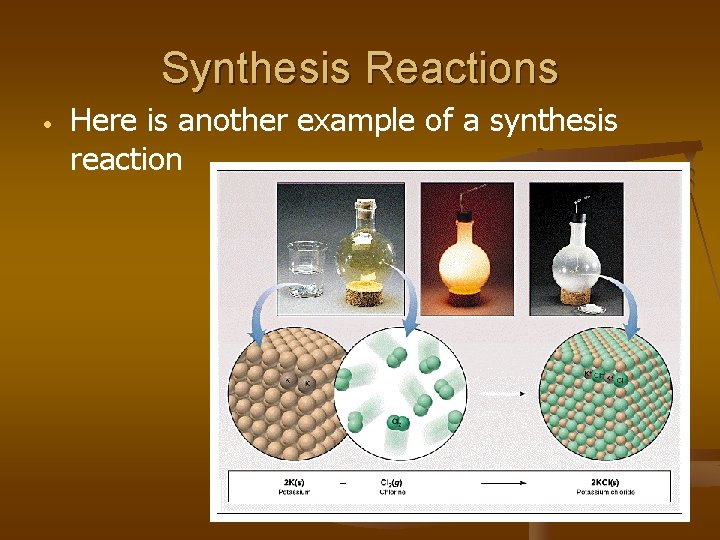
Synthesis Reactions • Here is another example of a synthesis reaction

Practice • • • Predict the products. Write and balance the following synthesis reaction equations. Sodium metal reacts with chlorine gas 2 Na(s) + Cl 2(g) 2 Na. Cl(s) Solid Magnesium reacts with fluorine gas Mg(s) + F 2(g) Mg. F 2(s)

2. Decomposition Reactions • • • Decomposition reactions occur when a compound breaks up into elements or two or more simpler compounds 1 Reactant Product + Product In general: AB A + B Example: 2 H 2 O 2 H 2 + O 2 Example: 2 Hg. O 2 Hg + O 2

Decomposition Reactions • Another view of a decomposition reaction:

Practice • • • Predict the products. Then, write and balance the following decomposition reaction equations: Solid Lead (IV) oxide decomposes Pb. O 2(s) Pb(s) + O 2(g) Aluminum nitride decomposes 2 Al. N(s) 2 Al(s) + N 2(g)

3. Single Replacement Reactions • • • Single Replacement Reactions occur when one element replaces another in a compound. A metal can replace a metal (+) OR a nonmetal can replace a nonmetal (-). element + compound product + product A + BC AC + B (if A is a metal) OR A + BC BA + C (if A is a nonmetal) (the positive ion always goes first!) When H 2 O splits into ions, it splits into H+ and OH- (not H+ and O-2 !!)

The Activity Series (see your Reference Sheet) n n n If the element doing the replacing is HIGHER in the series than the ion in the compound it is replacing, the REACTION WILL OCCUR. If the element doing the replacing is LOWER in the series than the ion in the compound it is replacing, the REACTION WILL NOT OCCUR. Li Rb K Ba Ca Na Mg Al Mn Zn Cr Fe Ni Sn Pb H Cu Hg Ag Pt Au Decreasing Activity Single Replacement Reactions F Cl Br I

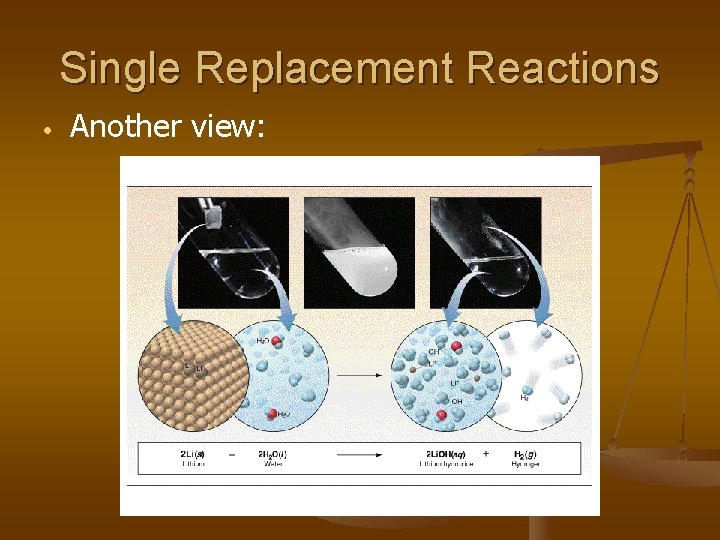
Single Replacement Reactions • Another view:

Single Replacement Reactions Write and balance the following single replacement reaction equation: • Zinc metal reacts with aqueous hydrochloric acid Zn(s) + 2 HCl(aq) Zn. Cl 2 + H 2(g) Note: Zinc replaces the hydrogen ion in the reaction •

Single Replacement Reactions • Aluminum metal reacts with aqueous copper (II) nitrate 2 Al(s)+ 3 Cu(NO 3)2(aq) 3 Cu(s) + 2 Al(NO 3)3(aq)

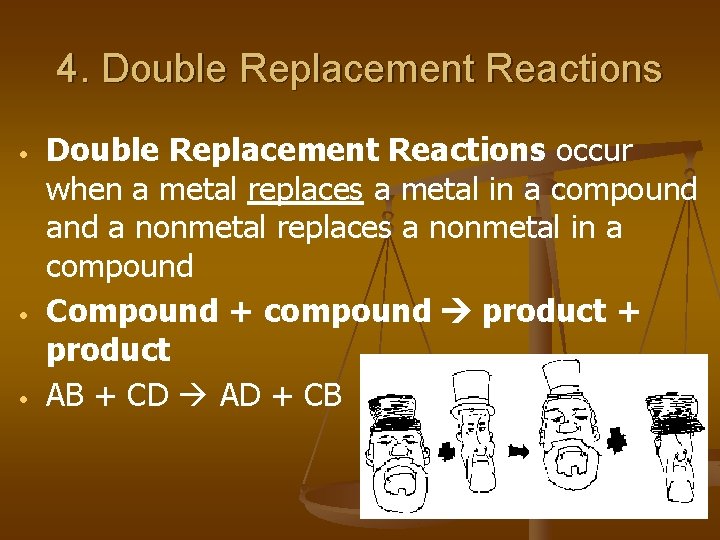
4. Double Replacement Reactions • • • Double Replacement Reactions occur when a metal replaces a metal in a compound a nonmetal replaces a nonmetal in a compound Compound + compound product + product AB + CD AD + CB

Solubility Rules A double replacement reaction between two compounds in aqueous solutions will only occur if a precipitate (an insoluble solid) is formed. (See Reference Sheet for Solubility Rules)

Solubility Rules n n n n n A product of a double replacement reaction will either be soluble (aq) or a precipitate (s) according to the rules below. 1. Nitrate (NO 3 -) – SOLUBLE 2. Alkali Metal Ions (Li+ , Na+ , K+ , Cs+ , Rb+) & Ammonium Ion (NH 4+) – SOLUBLE 3. Chloride (Cl-), bromide (Br-), and iodide (I-) salts – SOLUBLE Exceptions – salts containing the ions Ag+, Pb 2+, and Hg 22+ - INSOLUBLE 4. Sulfate (SO 42 -) salts - SOLUBLE Exceptions – Ba. SO 4 , Pb. SO 4 , Hg 2 SO 4, and Ca. SO 4 – INSOLUBLE 5. Most hydroxide (OH-) salts - slightly soluble Na. OH and KOH – SOLUBLE Ba(OH)2 , Sr(OH)2 , and Ca(OH)2 – marginally soluble 6. Sulfide (S 2 -) Carbonate (CO 32 -) Chromate (Cr. O 42 -) Phosphate (PO 43 -) salts -- slightly soluble

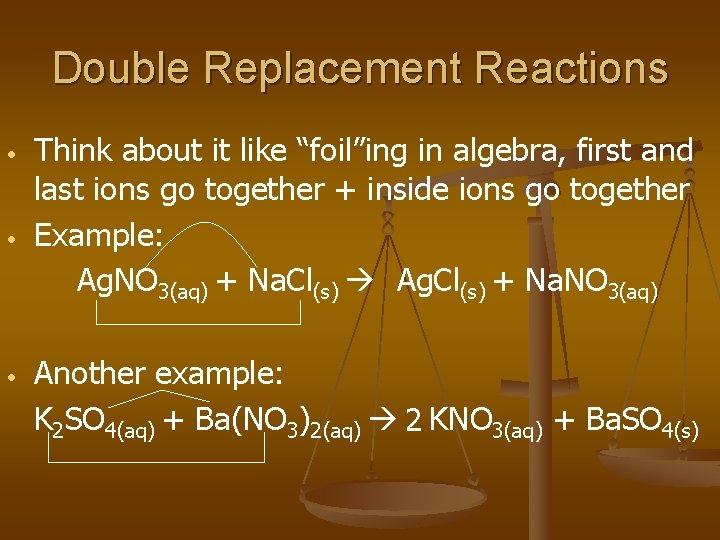
Double Replacement Reactions • • • Think about it like “foil”ing in algebra, first and last ions go together + inside ions go together Example: Ag. NO 3(aq) + Na. Cl(s) Ag. Cl(s) + Na. NO 3(aq) Another example: K 2 SO 4(aq) + Ba(NO 3)2(aq) 2 KNO 3(aq) + Ba. SO 4(s)

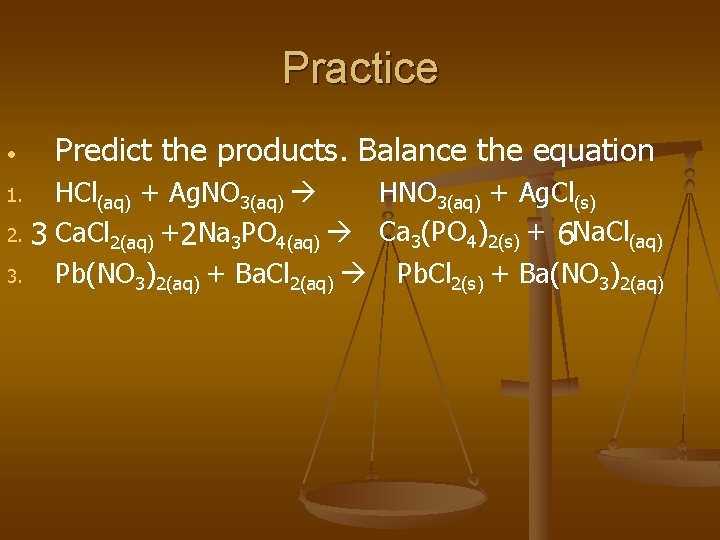
Practice • Predict the products. Balance the equation HNO 3(aq) + Ag. Cl(s) HCl(aq) + Ag. NO 3(aq) 2. 3 Ca. Cl 2(aq) +2 Na 3 PO 4(aq) Ca 3(PO 4)2(s) + 6 Na. Cl(aq) 3. Pb(NO 3)2(aq) + Ba. Cl 2(aq) Pb. Cl 2(s) + Ba(NO 3)2(aq) 1.

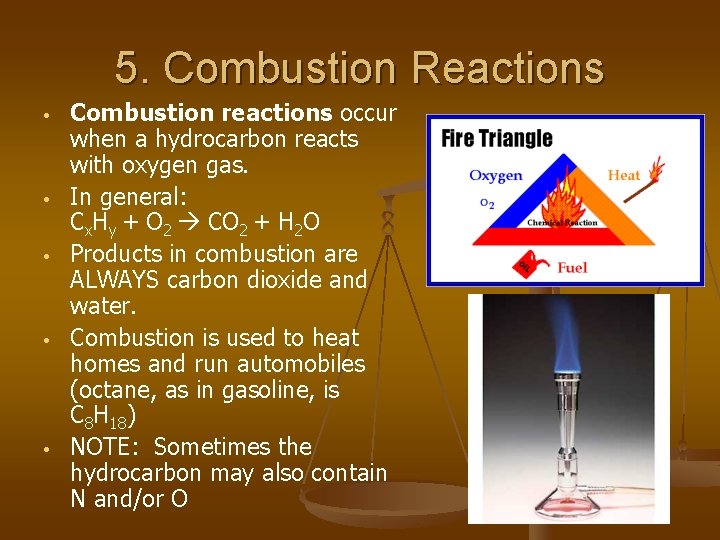
5. Combustion Reactions • • • Combustion reactions occur when a hydrocarbon reacts with oxygen gas. In general: Cx. Hy + O 2 CO 2 + H 2 O Products in combustion are ALWAYS carbon dioxide and water. Combustion is used to heat homes and run automobiles (octane, as in gasoline, is C 8 H 18) NOTE: Sometimes the hydrocarbon may also contain N and/or O

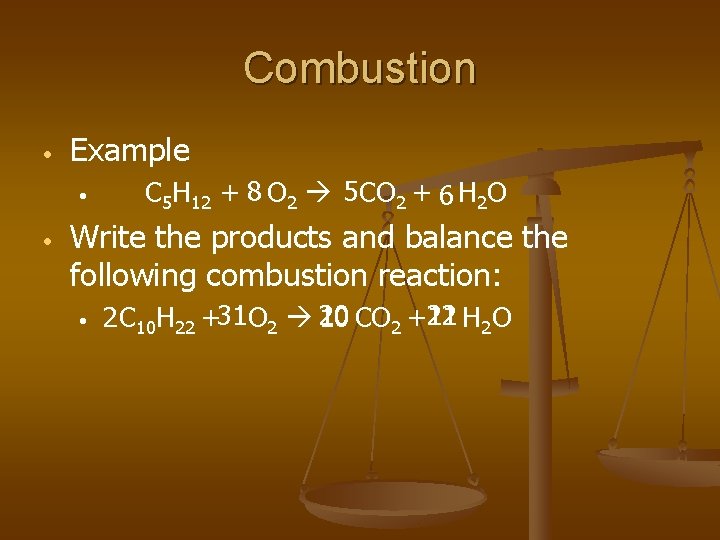
Combustion • Example • • C 5 H 12 + 8 O 2 5 CO 2 + 6 H 2 O Write the products and balance the following combustion reaction: • 11 H 2 O 2 C 10 H 22 +31 O 2 20 10 CO 2 +22

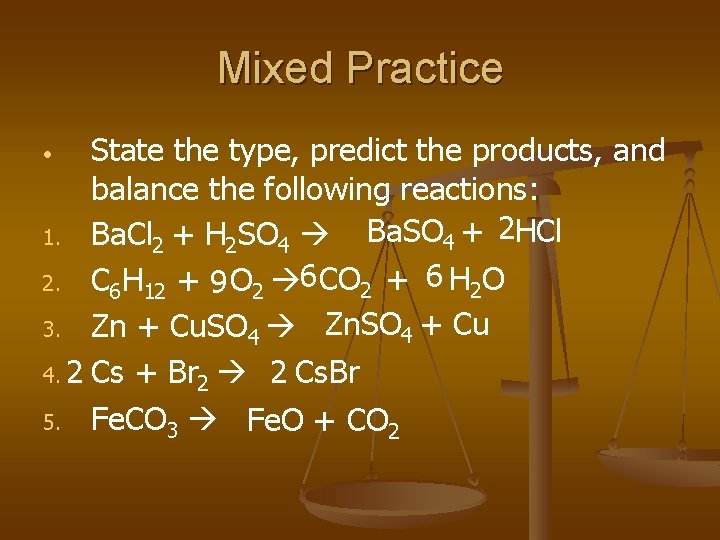
Mixed Practice State the type, predict the products, and balance the following reactions: Ba. SO 4 + 2 HCl 1. Ba. Cl 2 + H 2 SO 4 2. C 6 H 12 + 9 O 2 6 CO 2 + 6 H 2 O 3. Zn + Cu. SO 4 Zn. SO 4 + Cu 4. 2 Cs + Br 2 2 Cs. Br 5. Fe. CO 3 Fe. O + CO 2 •