Types of Reactions Synthesis reactions combine substances Example

Types of Reactions
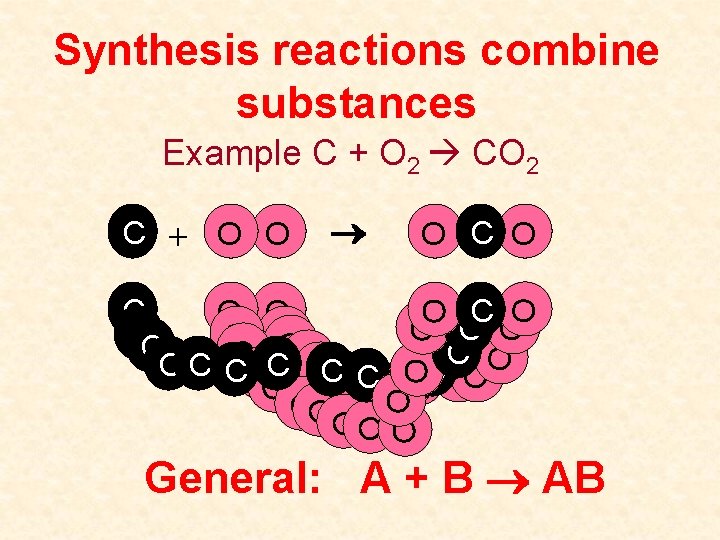
Synthesis reactions combine substances Example C + O 2 CO 2 C + O O O C O CC O O O C C C OC OOC C OCO OO CO O O OO OO OOO General: A + B AB
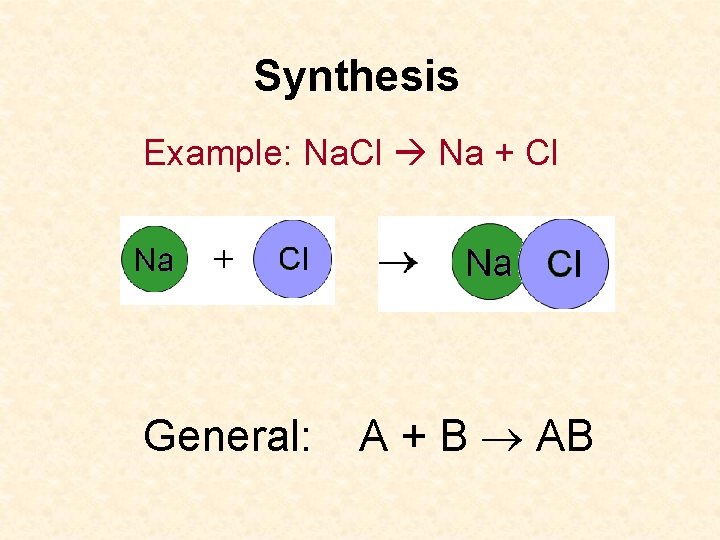
Synthesis Example: Na. Cl Na + Cl General: A + B AB
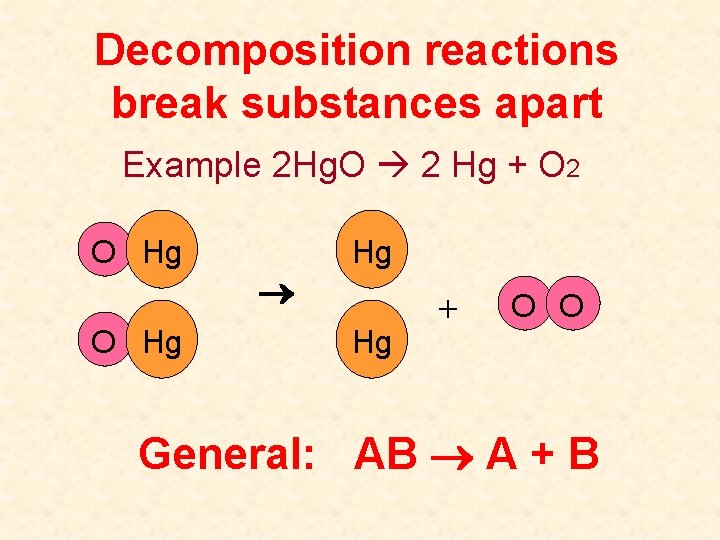
Decomposition reactions break substances apart Example 2 Hg. O 2 Hg + O 2 O Hg Hg Hg + O O General: AB A + B

Decomposition Examples • Digestion (breaking down food molecules into simpler substances) • Electrolysis of water – Electrolysis—the process by which an electric current is used to produce a chemical reaction – 2 H 20 (with electricity) 2 H 2 + O 2
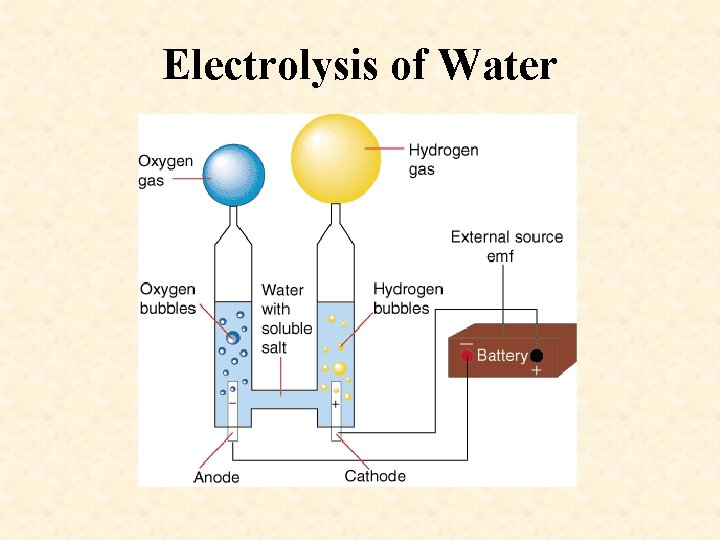
Electrolysis of Water
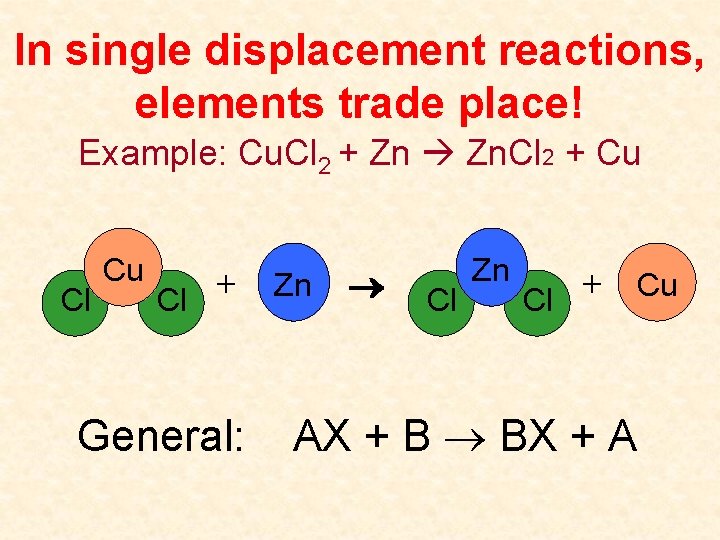
In single displacement reactions, elements trade place! Example: Cu. Cl 2 + Zn Zn. Cl 2 + Cu Cl Cu + Cl General: Zn Cl Zn + Cu Cl AX + B BX + A
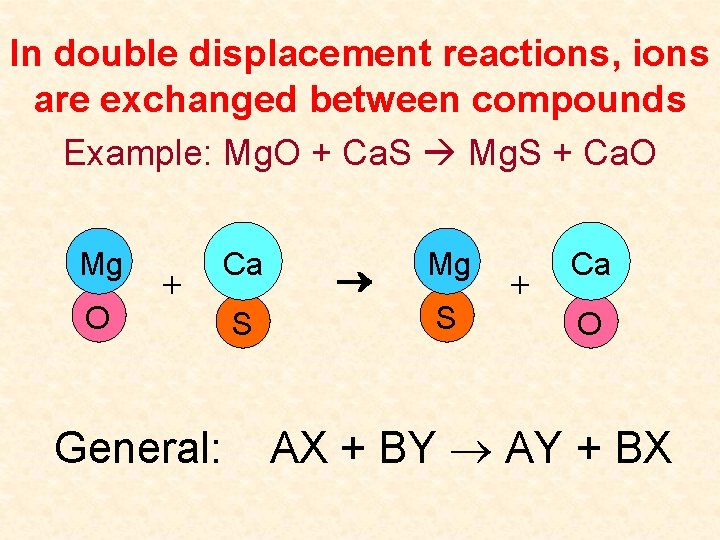
In double displacement reactions, ions are exchanged between compounds Example: Mg. O + Ca. S Mg. S + Ca. O Mg O + General: Ca S Mg S + Ca O AX + BY AY + BX
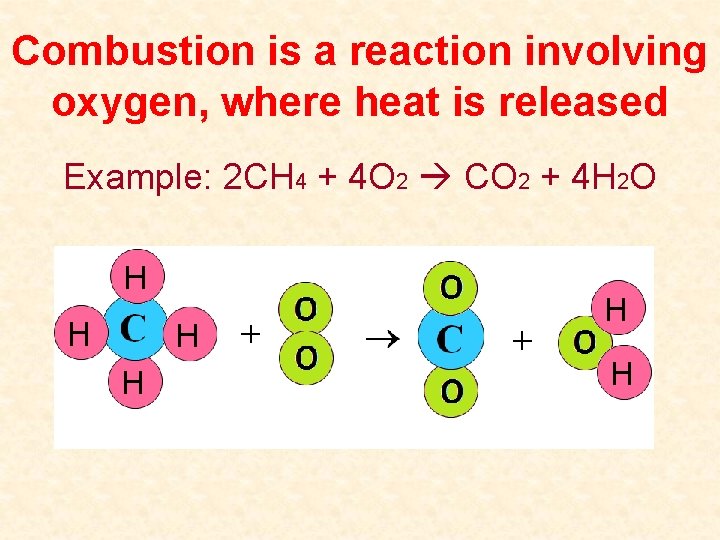
Combustion is a reaction involving oxygen, where heat is released Example: 2 CH 4 + 4 O 2 CO 2 + 4 H 2 O
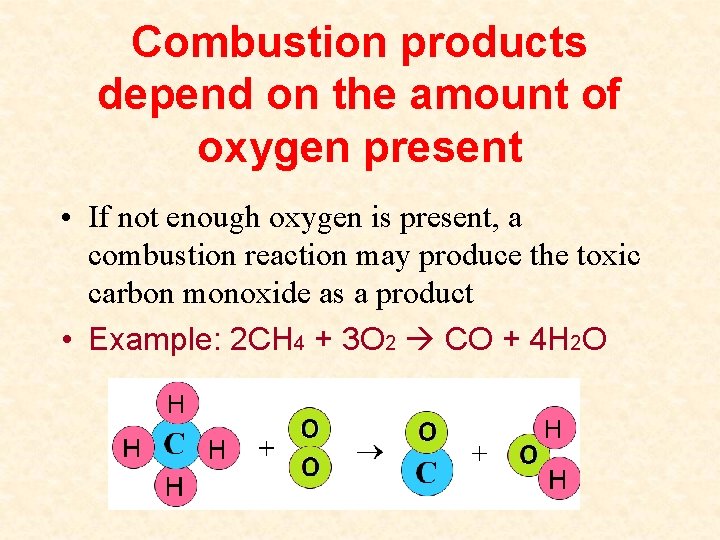
Combustion products depend on the amount of oxygen present • If not enough oxygen is present, a combustion reaction may produce the toxic carbon monoxide as a product • Example: 2 CH 4 + 3 O 2 CO + 4 H 2 O

Your favorite! • It’s good to hear it again though…
- Slides: 11