Types of Reactions Synthesis Reactions Also known as





- Slides: 13

Types of Reactions

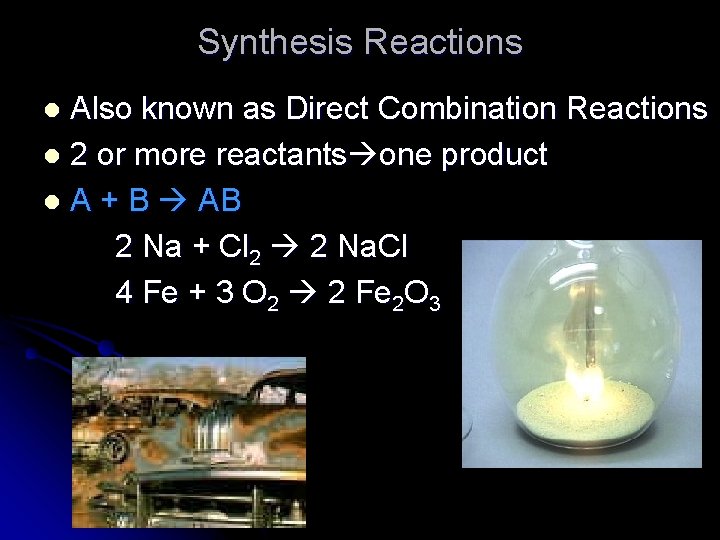
Synthesis Reactions Also known as Direct Combination Reactions l 2 or more reactants one product l A + B AB 2 Na + Cl 2 2 Na. Cl 4 Fe + 3 O 2 2 Fe 2 O 3 l

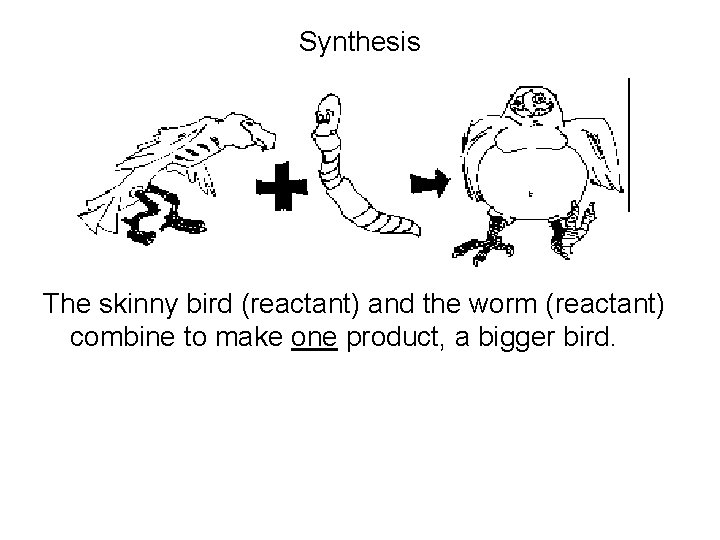
Synthesis The skinny bird (reactant) and the worm (reactant) combine to make one product, a bigger bird.

Decomposition Reactions l. A single compound broken down 2 or more smaller compounds or elements l Reverse of Synthesis Rxn l AB A + B 2 H 2 O 2 H 2 + O 2 Ca. CO 3 Ca. O + CO 2 (marble heated can break down)

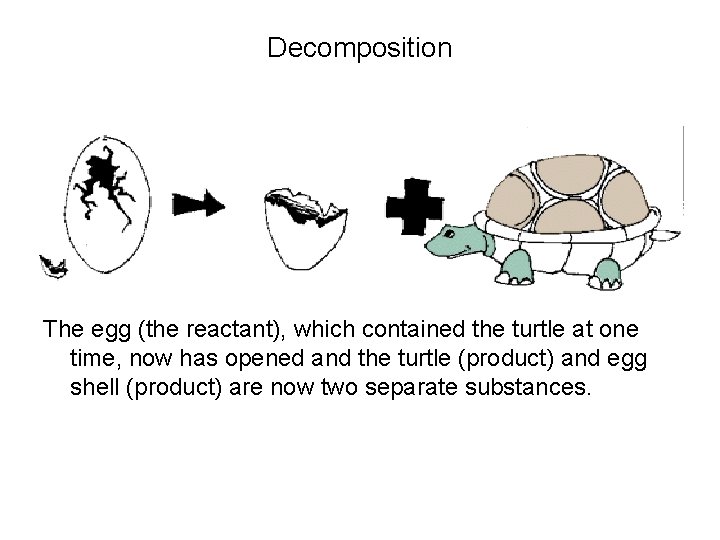
Decomposition The egg (the reactant), which contained the turtle at one time, now has opened and the turtle (product) and egg shell (product) are now two separate substances.

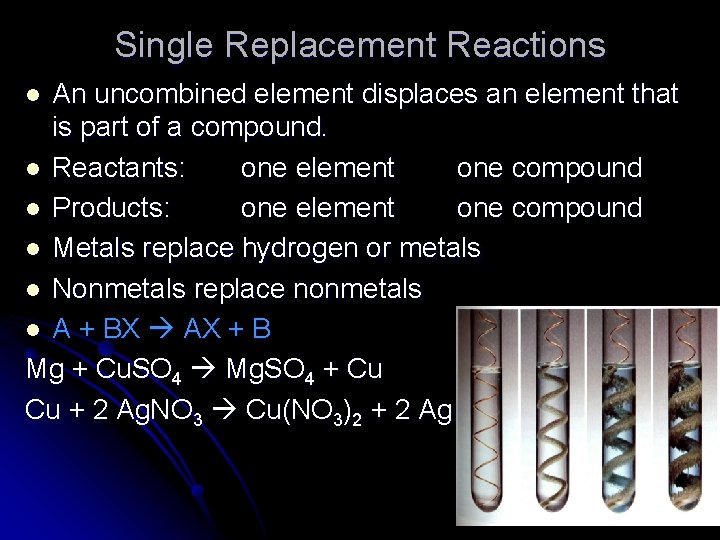
Single Replacement Reactions An uncombined element displaces an element that is part of a compound. l Reactants: one element one compound l Products: one element one compound l Metals replace hydrogen or metals l Nonmetals replace nonmetals l A + BX AX + B Mg + Cu. SO 4 Mg. SO 4 + Cu Cu + 2 Ag. NO 3 Cu(NO 3)2 + 2 Ag l

Single Replacement Reactions Not all elements can replace all other elements. l A more active element will replace a less active element l Activity Series (text p 288) Metals most active Li ↓ K ↓ Ba l Li can replace K or Ba l Can Ba replace K? l

Single Replacement

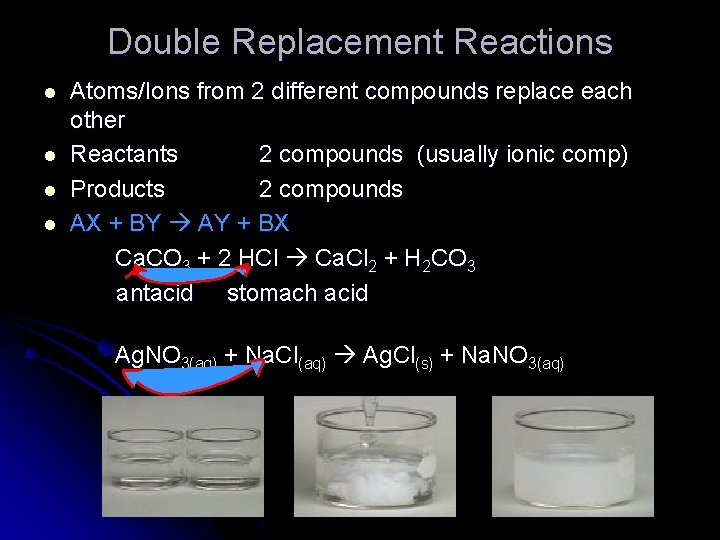
Double Replacement Reactions l l Atoms/Ions from 2 different compounds replace each other Reactants 2 compounds (usually ionic comp) Products 2 compounds AX + BY AY + BX Ca. CO 3 + 2 HCl Ca. Cl 2 + H 2 CO 3 antacid stomach acid Ag. NO 3(aq) + Na. Cl(aq) Ag. Cl(s) + Na. NO 3(aq)

Double Replacement

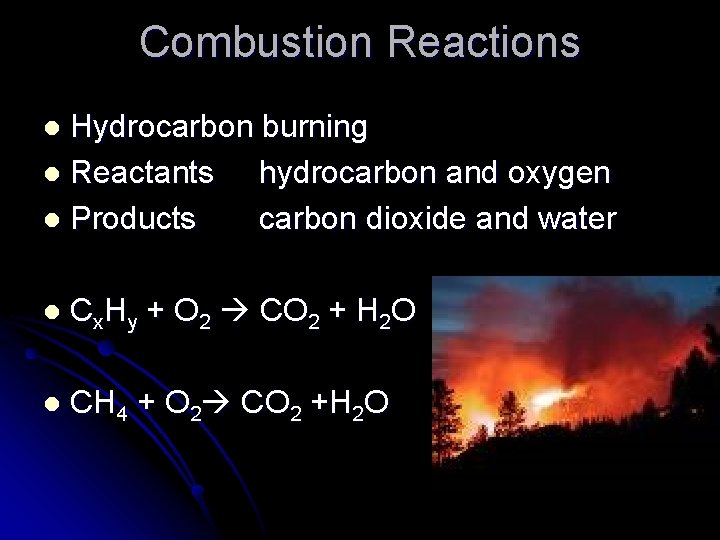
Combustion Reactions Hydrocarbon burning l Reactants hydrocarbon and oxygen l Products carbon dioxide and water l l Cx. Hy + O 2 CO 2 + H 2 O l CH 4 + O 2 CO 2 +H 2 O

Quiz 11 -14 1. Aluminum lawn furniture becomes coated with a layer of aluminum oxide when it sits out in the air. 2. Chlorine gas is bubbled through a calcium bromide solution. The solution turns brown, the color of bromine. 3. Lime (Ca. O) is added to acidic water in a lake, water and salt are formed. 4. Propane is a common household fuel. When burned, water and carbon dioxide are produced. 5. When an electric current is passed through molten potassium bromide, potassium and bromine form.

Quiz 11 -9 Magnesium reacts with aqueous hydrochloric acid to form magnesium chloride solution and hydrogen gas. 1. 2. Write a balanced equation for the reaction. What type of reaction is it?