Types of Reactions Synthesis Decomposition Single Replacement Double
Types of Reactions • • • Synthesis Decomposition Single Replacement Double Replacement Combustion
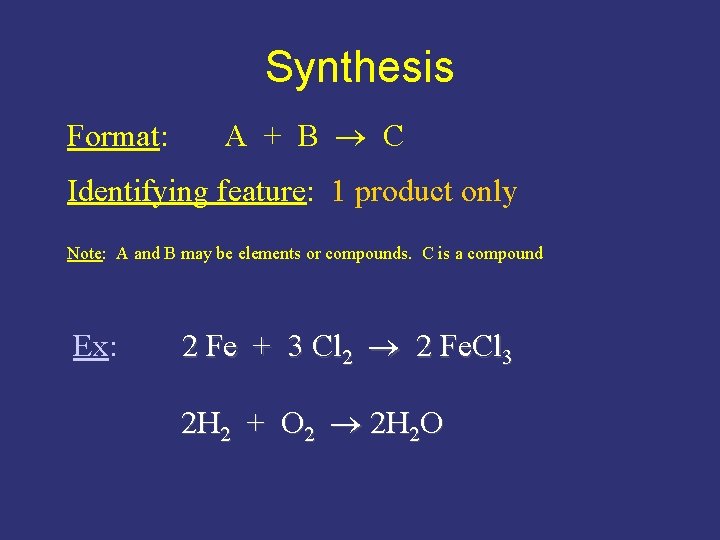
Synthesis Format: A + B C Identifying feature: 1 product only Note: A and B may be elements or compounds. C is a compound Ex: 2 Fe + 3 Cl 2 2 Fe. Cl 3 2 H 2 + O 2 2 H 2 O
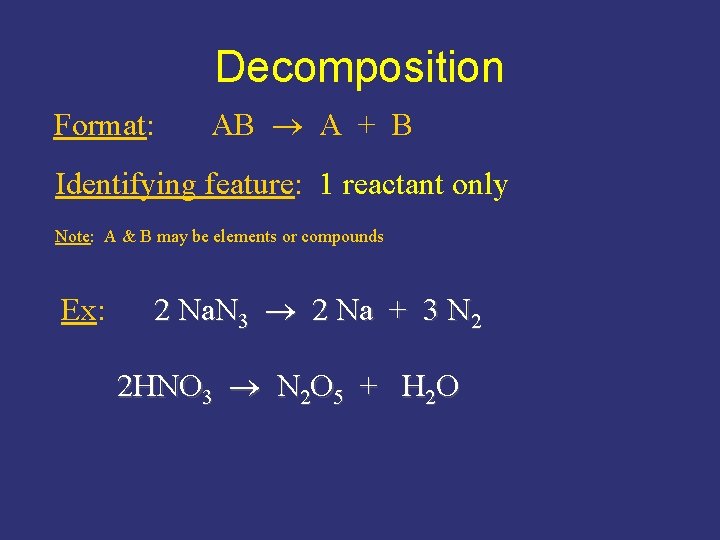
Decomposition Format: AB A + B Identifying feature: 1 reactant only Note: A & B may be elements or compounds Ex: 2 Na. N 3 2 Na + 3 N 2 2 HNO 3 N 2 O 5 + H 2 O
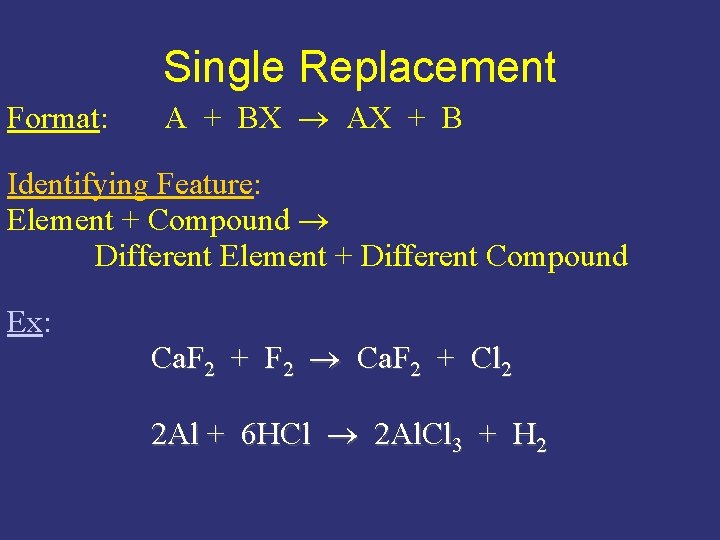
Single Replacement Format: A + BX AX + B Identifying Feature: Element + Compound Different Element + Different Compound Ex: Ca. F 2 + F 2 Ca. F 2 + Cl 2 2 Al + 6 HCl 2 Al. Cl 3 + H 2
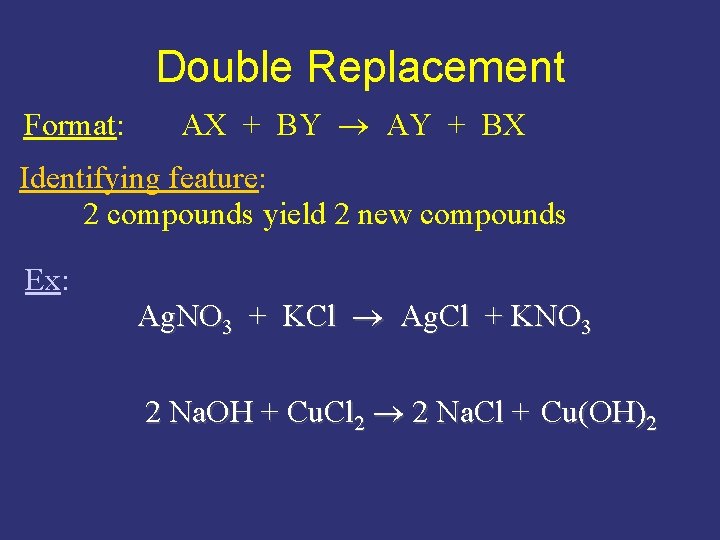
Double Replacement Format: AX + BY AY + BX Identifying feature: 2 compounds yield 2 new compounds Ex: Ag. NO 3 + KCl Ag. Cl + KNO 3 2 Na. OH + Cu. Cl 2 2 Na. Cl + Cu(OH)2
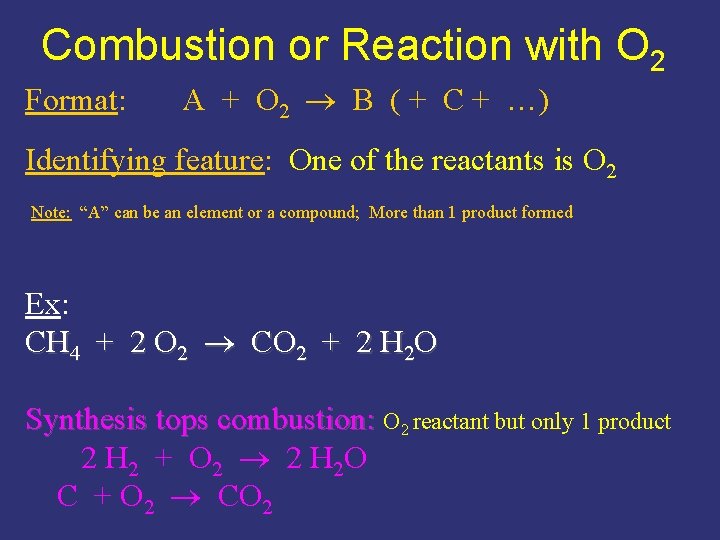
Combustion or Reaction with O 2 Format: A + O 2 B ( + C + …) Identifying feature: One of the reactants is O 2 Note: “A” can be an element or a compound; More than 1 product formed Ex: CH 4 + 2 O 2 CO 2 + 2 H 2 O Synthesis tops combustion: O 2 reactant but only 1 product 2 H 2 + O 2 2 H 2 O C + O 2 CO 2
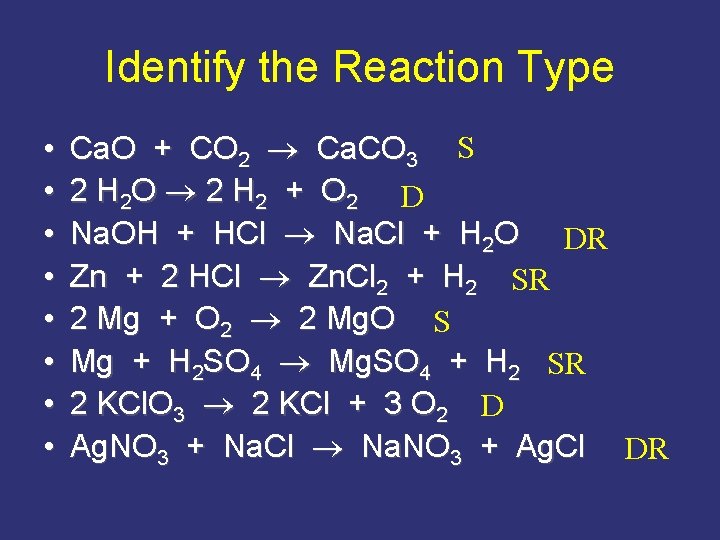
Identify the Reaction Type • • Ca. O + CO 2 Ca. CO 3 S 2 H 2 O 2 H 2 + O 2 D Na. OH + HCl Na. Cl + H 2 O DR Zn + 2 HCl Zn. Cl 2 + H 2 SR 2 Mg + O 2 2 Mg. O S Mg + H 2 SO 4 Mg. SO 4 + H 2 SR 2 KCl. O 3 2 KCl + 3 O 2 D Ag. NO 3 + Na. Cl Na. NO 3 + Ag. Cl DR
- Slides: 7