TYPES OF REACTIONS SYNTHESIS COMBINATION REACTION two or

TYPES OF REACTIONS

SYNTHESIS (COMBINATION) REACTION • two or more substances combine to form a new compound • reaction is represented by the following equation. • A +B AB
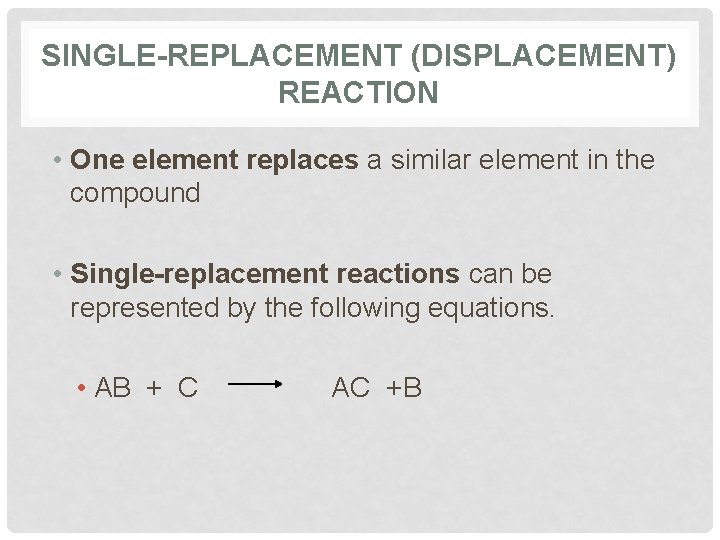
SINGLE-REPLACEMENT (DISPLACEMENT) REACTION • One element replaces a similar element in the compound • Single-replacement reactions can be represented by the following equations. • AB + C AC +B

DOUBLE-REPLACEMENT REACTION • ions of two compounds exchange places in an aqueous solution to form two new compounds. • A double-replacement reaction can be represented by the following equation. • AB + CD AC + BD

DECOMPOSITION REACTION • single compound undergoes a reaction that produces two or more simpler substances. • A decomposition reaction can be represented by the following equation. • AB A + B
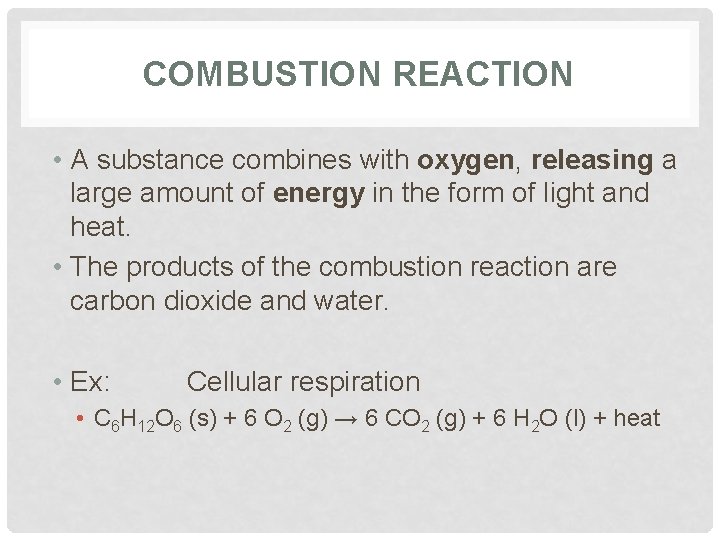
COMBUSTION REACTION • A substance combines with oxygen, releasing a large amount of energy in the form of light and heat. • The products of the combustion reaction are carbon dioxide and water. • Ex: Cellular respiration • C 6 H 12 O 6 (s) + 6 O 2 (g) → 6 CO 2 (g) + 6 H 2 O (l) + heat

STANDARD SC. 912. P. 8. 8 Characterize types of chemical reactions, for example: redox, acid‐base, synthesis, and single and double replacement reactions. • identify a reaction as synthesis, replacement (single displacement and double displacement), decomposition, or combustion
- Slides: 7