Types of reactions RICE Chemical Reactions Indications that






- Slides: 24

Types of reactions RICE

Chemical Reactions

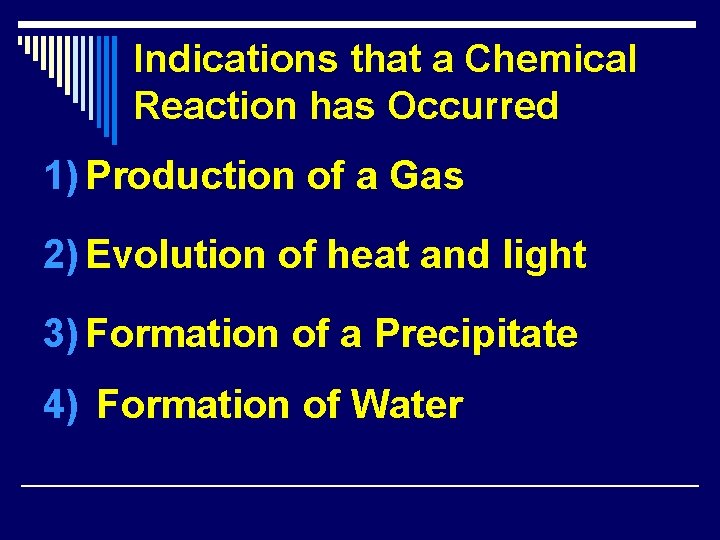
Indications that a Chemical Reaction has Occurred 1) Production of a Gas 2) Evolution of heat and light 3) Formation of a Precipitate 4) Formation of Water

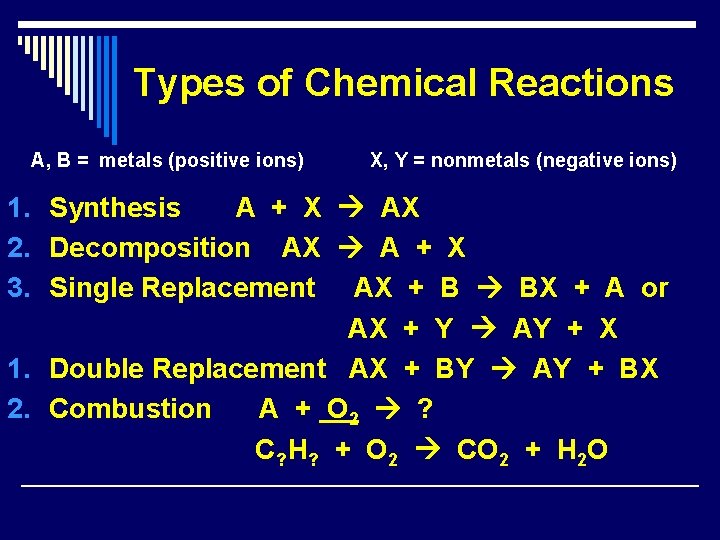
Types of Chemical Reactions A, B = metals (positive ions) X, Y = nonmetals (negative ions) 1. Synthesis A + X AX 2. Decomposition AX A + X 3. Single Replacement AX + B BX + A or AX + Y AY + X 1. Double Replacement AX + BY AY + BX 2. Combustion A + O 2 ? C? H? + O 2 CO 2 + H 2 O

Predicting Reactions Synthesis A + X AX

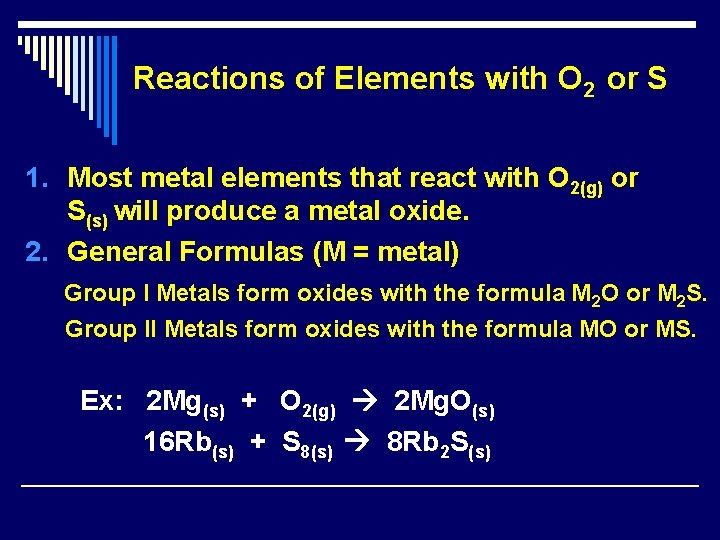
Reactions of Elements with O 2 or S 1. Most metal elements that react with O 2(g) or S(s) will produce a metal oxide. 2. General Formulas (M = metal) Group I Metals form oxides with the formula M 2 O or M 2 S. Group II Metals form oxides with the formula MO or MS. Ex: 2 Mg(s) + O 2(g) 2 Mg. O(s) 16 Rb(s) + S 8(s) 8 Rb 2 S(s)

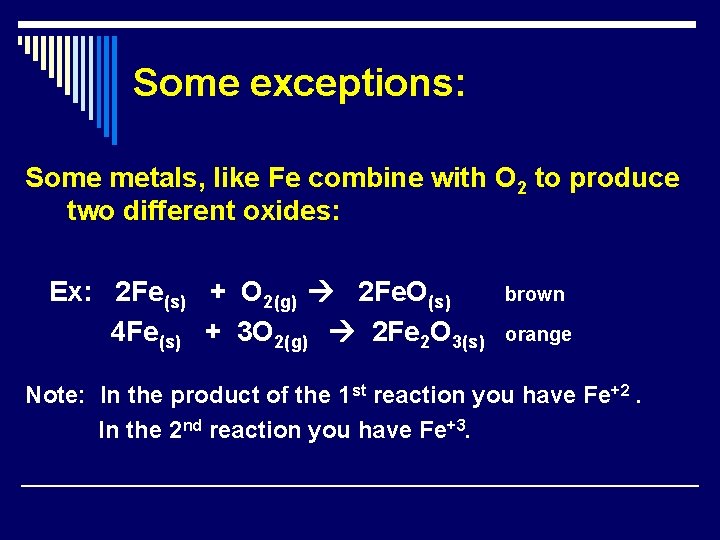
Some exceptions: Some metals, like Fe combine with O 2 to produce two different oxides: Ex: 2 Fe(s) + O 2(g) 2 Fe. O(s) 4 Fe(s) + 3 O 2(g) 2 Fe 2 O 3(s) brown orange Note: In the product of the 1 st reaction you have Fe+2. In the 2 nd reaction you have Fe+3.

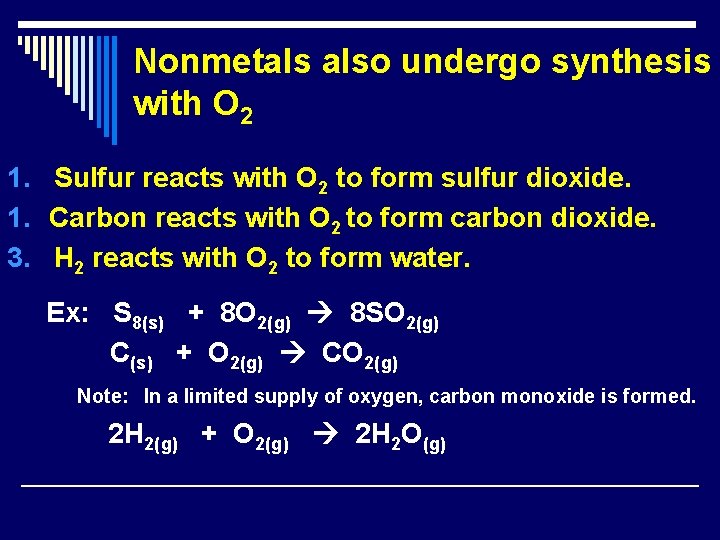
Nonmetals also undergo synthesis with O 2 1. Sulfur reacts with O 2 to form sulfur dioxide. 1. Carbon reacts with O 2 to form carbon dioxide. 3. H 2 reacts with O 2 to form water. Ex: S 8(s) + 8 O 2(g) 8 SO 2(g) C(s) + O 2(g) CO 2(g) Note: In a limited supply of oxygen, carbon monoxide is formed. 2 H 2(g) + O 2(g) 2 H 2 O(g)

More Synthesis Reactions

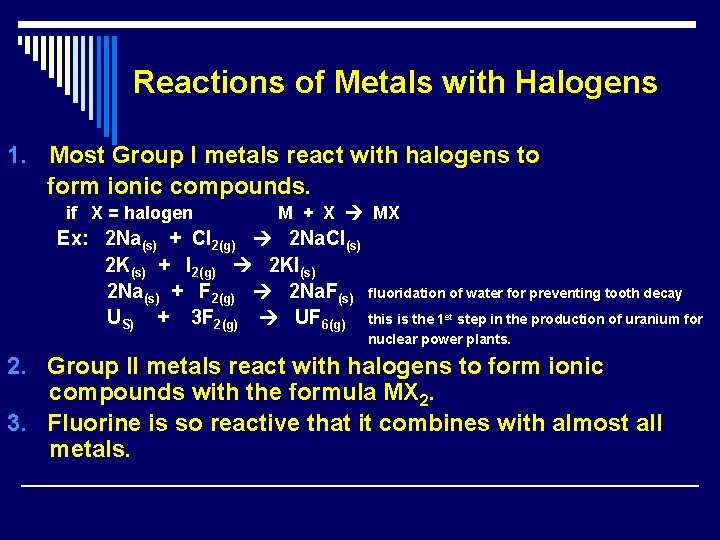
Reactions of Metals with Halogens 1. Most Group I metals react with halogens to form ionic compounds. if X = halogen M + X MX Ex: 2 Na(s) + Cl 2(g) 2 Na. Cl(s) 2 K(s) + I 2(g) 2 KI(s) 2 Na(s) + F 2(g) 2 Na. F(s) US) + 3 F 2(g) UF 6(g) fluoridation of water for preventing tooth decay this is the 1 st step in the production of uranium for nuclear power plants. 2. Group II metals react with halogens to form ionic compounds with the formula MX 2. 3. Fluorine is so reactive that it combines with almost all metals.

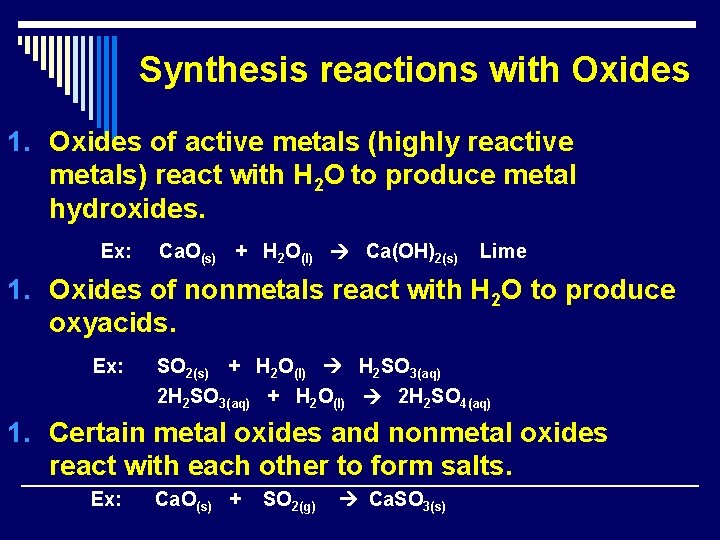
Synthesis reactions with Oxides 1. Oxides of active metals (highly reactive metals) react with H 2 O to produce metal hydroxides. Ex: Ca. O(s) + H 2 O(l) Ca(OH)2(s) Lime 1. Oxides of nonmetals react with H 2 O to produce oxyacids. Ex: SO 2(s) + H 2 O(l) H 2 SO 3(aq) 2 H 2 SO 3(aq) + H 2 O(l) 2 H 2 SO 4(aq) 1. Certain metal oxides and nonmetal oxides react with each other to form salts. Ex: Ca. O(s) + SO 2(g) Ca. SO 3(s)

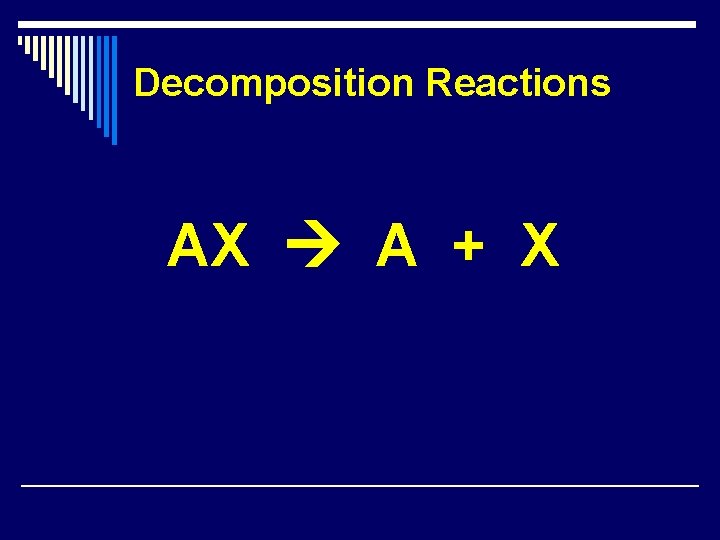
Decomposition Reactions AX A + X

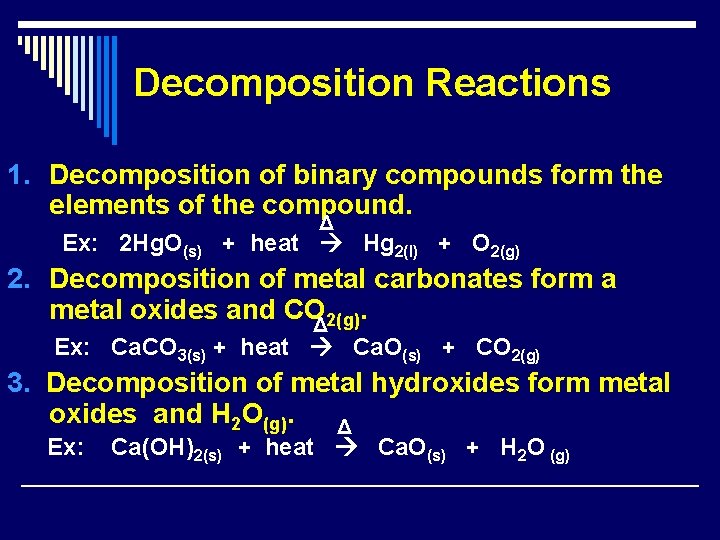
Decomposition Reactions 1. Decomposition of binary compounds form the elements of the compound. Δ Ex: 2 Hg. O(s) + heat Hg 2(l) + O 2(g) 2. Decomposition of metal carbonates form a metal oxides and COΔ 2(g). Ex: Ca. CO 3(s) + heat Ca. O(s) + CO 2(g) 3. Decomposition of metal hydroxides form metal oxides and H 2 O(g). Δ Ex: Ca(OH)2(s) + heat Ca. O(s) + H 2 O (g)

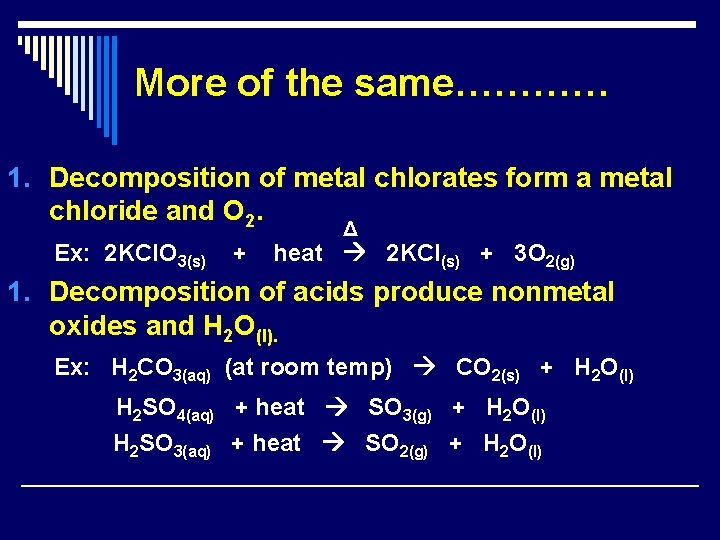
More of the same………… 1. Decomposition of metal chlorates form a metal chloride and O 2. Ex: 2 KCl. O 3(s) + Δ heat 2 KCl(s) + 3 O 2(g) 1. Decomposition of acids produce nonmetal oxides and H 2 O(l). Ex: H 2 CO 3(aq) (at room temp) CO 2(s) + H 2 O(l) H 2 SO 4(aq) + heat SO 3(g) + H 2 O(l) H 2 SO 3(aq) + heat SO 2(g) + H 2 O(l)

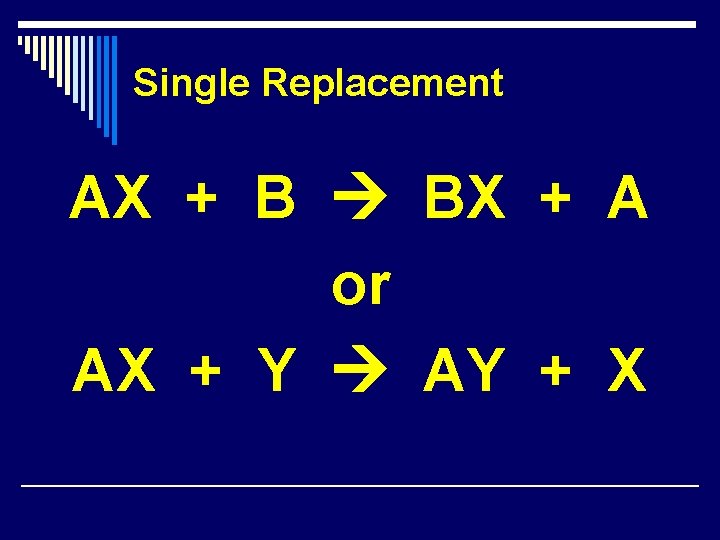
Single Replacement AX + B BX + A or AX + Y AY + X

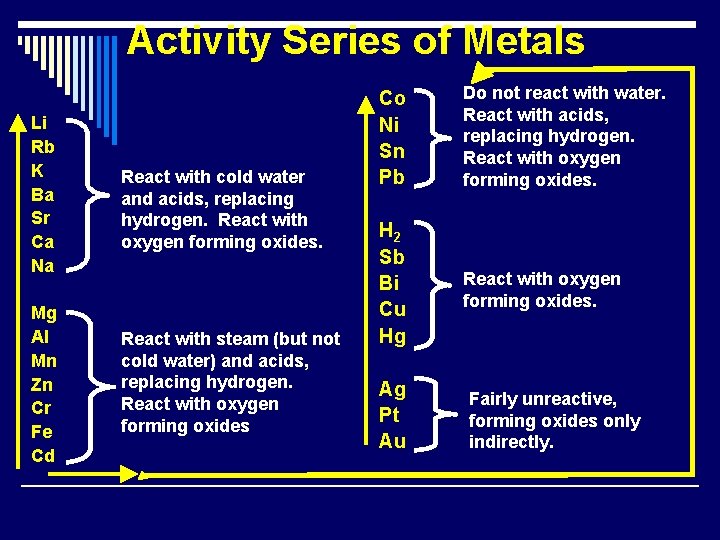
Activity Series A list of elements organized according to the ease with which the elements undergo certain chemical reactions. Ø Used in single replacement reactions to predict if a chemical reaction will occur. Ø You will need to memorize these!

Activity Series of Metals Li Rb K Ba Sr Ca Na Mg Al Mn Zn Cr Fe Cd React with cold water and acids, replacing hydrogen. React with oxygen forming oxides. React with steam (but not cold water) and acids, replacing hydrogen. React with oxygen forming oxides Co Ni Sn Pb Do not react with water. React with acids, replacing hydrogen. React with oxygen forming oxides. H 2 Sb Bi Cu Hg React with oxygen forming oxides. Ag Pt Au Fairly unreactive, forming oxides only indirectly.

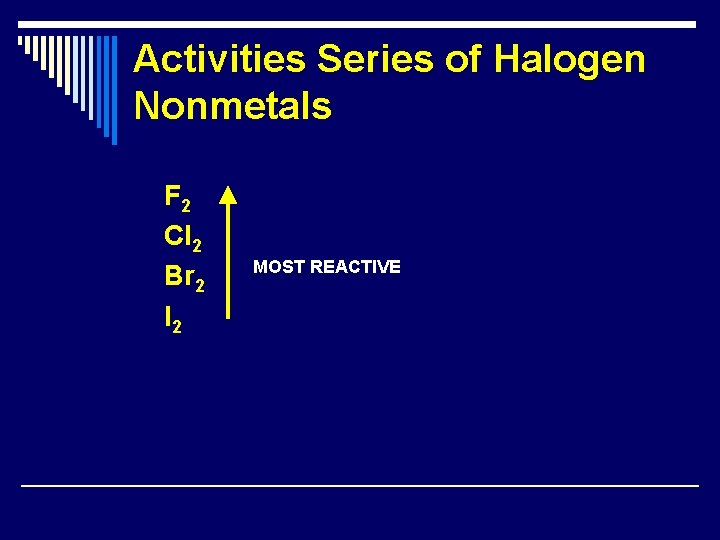
Activities Series of Halogen Nonmetals F 2 Cl 2 Br 2 I 2 MOST REACTIVE

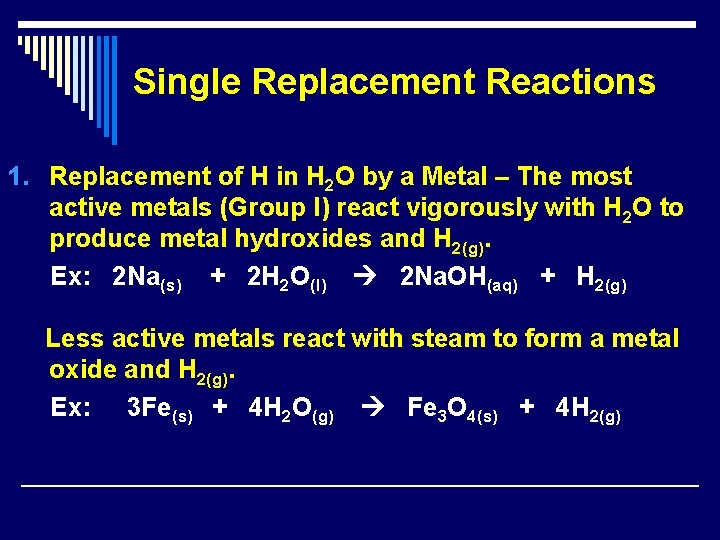
Single Replacement Reactions 1. Replacement of H in H 2 O by a Metal – The most active metals (Group I) react vigorously with H 2 O to produce metal hydroxides and H 2(g). Ex: 2 Na(s) + 2 H 2 O(l) 2 Na. OH(aq) + H 2(g) Less active metals react with steam to form a metal oxide and H 2(g). Ex: 3 Fe(s) + 4 H 2 O(g) Fe 3 O 4(s) + 4 H 2(g)

More on Single Replacement 1. Replacement of H in an acid by a metal -- The more active metals react with certain acidic solutions (H 2 SO 4 and HCl) to form a salt and H 2(g). Ex: Mg(s) + 2 HCl(aq) H 2(g) + Mg. Cl 2(aq) 3. Replacement of Halogens – When one halogen replaces another halogen the activity series is used to determine if a reaction will occur. The most active halogen is F 2. The activity decreases as you go down the halogen group in the periodic table.

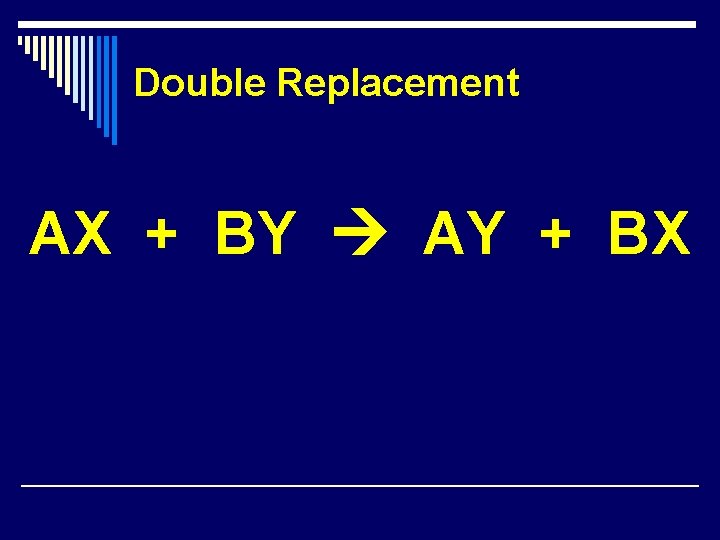
Double Replacement AX + BY AY + BX

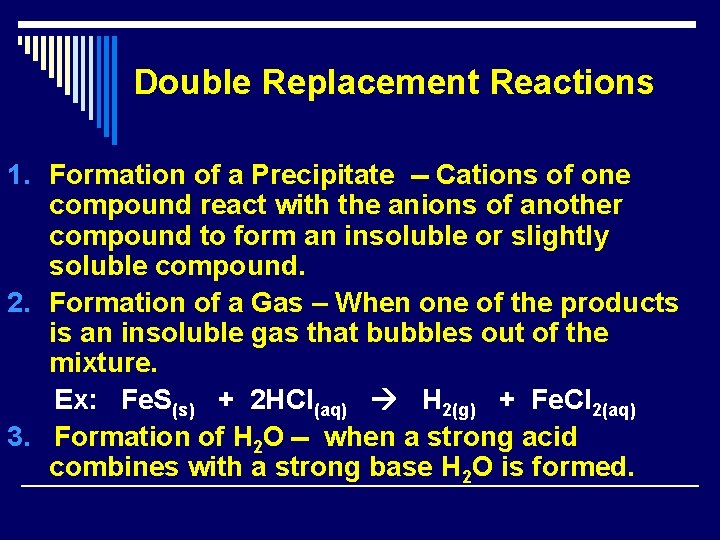
Double Replacement Reactions 1. Formation of a Precipitate -- Cations of one compound react with the anions of another compound to form an insoluble or slightly soluble compound. 2. Formation of a Gas – When one of the products is an insoluble gas that bubbles out of the mixture. Ex: Fe. S(s) + 2 HCl(aq) H 2(g) + Fe. Cl 2(aq) 3. Formation of H 2 O -- when a strong acid combines with a strong base H 2 O is formed.

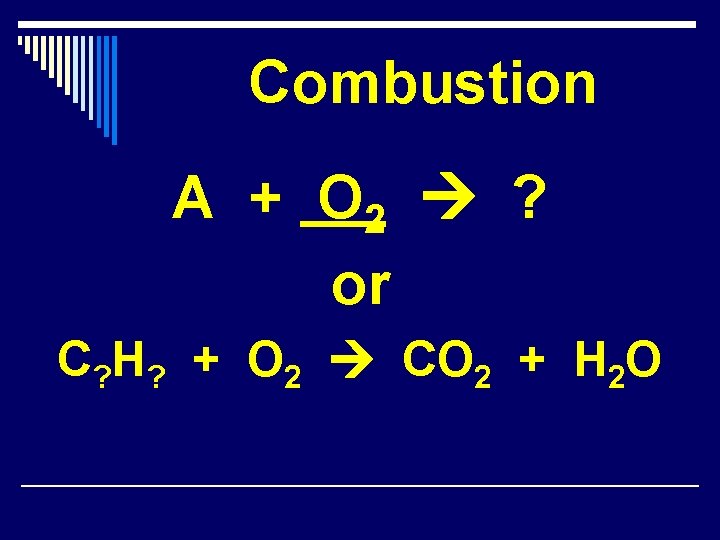
Combustion A + O 2 ? or C? H? + O 2 CO 2 + H 2 O

Combustion Reactions 1. A hydrocarbon plus O 2(g) will always equal CO 2(g) and H 2 O(g). Ex: C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + H 2 O(g) 1. The combustion of H 2(g) yields water vapor. Ex: 2 H 2(g) + O 2(g) 2 H 2 O(g)