Types of Reactions I Synthesis reactions have only













- Slides: 21

Types of Reactions I. Synthesis reactions – have only one product. General Form: A + X AX EX: • 2 Na(s) + Cl 2(g) 2 Na. Cl(s) • 2 H 2(g) + O 2(g) 2 H 2 O(l) • Ca. O(s) + CO 2(g) Ca. CO 3(s) *Metals combine with non-metals in synthesis rxns to form ionic compounds. EX: Mg + Br 2 Mg. Br 2

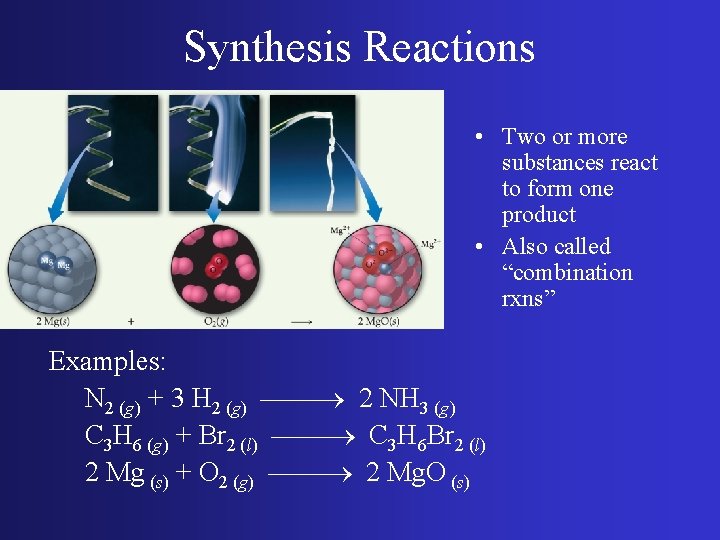
Synthesis Reactions • Two or more substances react to form one product • Also called “combination rxns” Examples: N 2 (g) + 3 H 2 (g) 2 NH 3 (g) C 3 H 6 (g) + Br 2 (l) C 3 H 6 Br 2 (l) 2 Mg (s) + O 2 (g) 2 Mg. O (s)

Practice • Predict the products then write and balance the following synthesis reaction equations. • Sodium metal reacts with chlorine gas Na(s) + Cl 2(g) • Solid Magnesium reacts with fluorine gas Mg(s) + F 2(g) • Aluminum metal reacts with fluorine gas Al(s) + F 2(g)

II. Decomposition Reactions • Have only one reactant • General Form: AX A + X • One substance breaks down into two or more substances • Examples: Ca. CO 3 (s) Ca. O (s) + CO 2 (g) 2 KCl. O 3 (s) 2 KCl (s) + O 2 (g) 2 Na. N 3 (s) 2 Na (s) + 3 N 2 (g)

Electrolysis – decomposing a compound by running an electric current through it. 2 H 2 O 2 H 2 + O 2

* The decomposition of binary compounds produces the two elements in the compound. EX: 2 Na. Cl(l) 2 Na(s) + Cl 2(g) N 2 O 4 N 2 + 2 O 2 Special decomposition rxns: a) Decomposition of metal carbonates produces the metal oxide and CO 2 EX: Mg. CO 3 Mg. O + CO 2 Write the equation for the decomposition of Na 2 CO 3 Na 2 O + CO 2

b. Decomposition of metal hydroxides produce the metal oxide and water. EX: Mg(OH)2 Mg. O + H 2 O Write the equation for the decomposition of KOH. 2 KOH K 2 O + H 2 O

c. Carbonic acid rapidly decomposes upon its formation: H 2 CO 3(aq) H 2 O(l) + CO 2(g)

Practice • Predict the products then write and balance the following decomposition rxn equations: • Solid Lead (IV) oxide decomposes Pb. O 2(s) • Aluminum nitride decomposes Al. N(s) • Calcium carbonate is decomposed by heating Ca. CO 3 • Copper(II) hydroxide is decomposed Cu(OH)2

III. Single Replacement Reactions An element replaces another element in a compound. General forms: A + BX B + AX (metal takes place of metal) or Y + BX X + BY (nonmetal takes place of nonmetal) These rxns usually occur in solution.

EX: Cu(s)+ 2 Ag. NO 3(aq) 2 Ag(s) + Cu(NO 3)2(aq) Cl 2(g) + 2 Na. Br 2(aq) Br 2(l) + 2 Na. Cl(aq)

*** For a single replacement rxn to occur, an element must be more reactive than the element it replaces*** See the “Activity Series of Elements” on the back of your periodic table. In order for an element to replace another element in a compound, it must be higher than that element on the activity series. Cu(s)+ 2 Ag. NO 3(aq) 2 Ag(s) + Cu(NO 3)2(aq) Occurs - Copper is more reactive than silver Cl 2(g) + 2 Na. Br 2(aq) Br 2(l) + 2 Na. Cl(aq) Occurs - Chlorine is more reactive than bromine

Al(s) + Na. Cl(aq) No Rxn Aluminum is LESS reactive than sodium. Br 2(l) + Na. Cl(aq) No Rxn Bromine is LESS reactive than chlorine ----------------------------Remember that metals replace metals, and nonmetals replace nonmetals.

If the following rxns occur, write the products and balance the equation; if no reaction occurs, write “No Rxn” 1. Mg(s) + Zn. Cl 2(aq) Zn(s) + Mg. Cl 2(aq) 2. 2 Al(s) + 3 Pb(NO 3)2(aq) 3 Pb(s) +2 Al(NO 3)3(aq) 3. Fe(s) + Al. Cl 3(aq) No Rxn 4. I 2(s) + Na. Cl(aq) No Rxn 5. F 2(g) + 2 Li. Br(aq) Br 2(l) + 2 Li. F(aq)

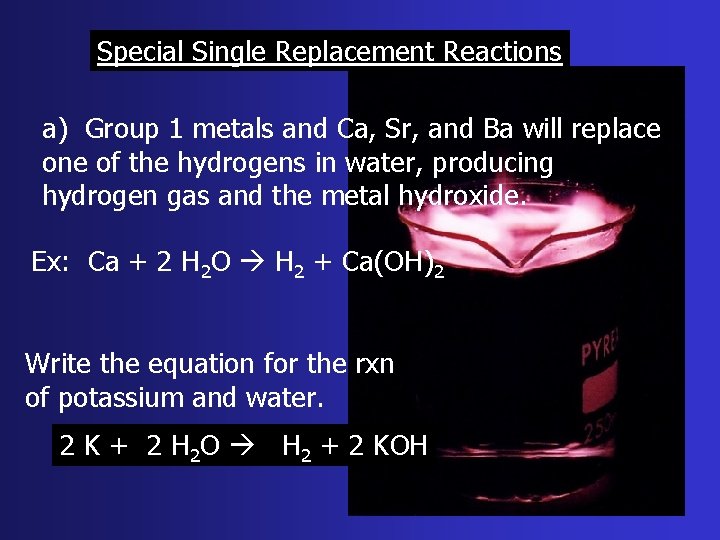
Special Single Replacement Reactions a) Group 1 metals and Ca, Sr, and Ba will replace one of the hydrogens in water, producing hydrogen gas and the metal hydroxide. Ex: Ca + 2 H 2 O H 2 + Ca(OH)2 Write the equation for the rxn of potassium and water. 2 K + 2 H 2 O H 2 + 2 KOH

b) Metals can replace the hydrogen in acids. Ex: 2 Al(s) + 6 HCl(aq) 3 H 2(g) + 2 Al. Cl 3(aq) *note that hydrogen is listed with the metals on the Activity Series Write the equation for the rxn between nitric acid and magnesium metal. Mg + 2 HNO 3 H 2 + Mg(NO 3)2

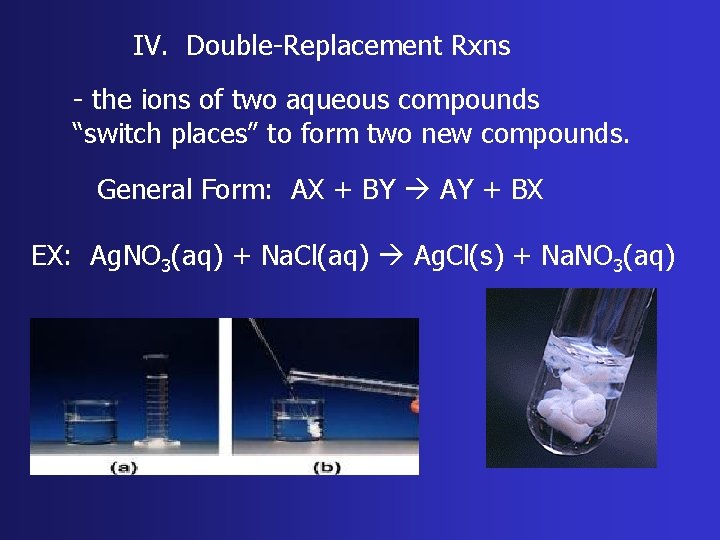
IV. Double-Replacement Rxns - the ions of two aqueous compounds “switch places” to form two new compounds. General Form: AX + BY AY + BX EX: Ag. NO 3(aq) + Na. Cl(aq) Ag. Cl(s) + Na. NO 3(aq)

In order for a double replacement rxn to occur, one of the products must remove its ions from aqueous solution. This can occur when: 1) One of the products is insoluble (forms a solid) in water. A precipitate forms. See the solubility chart on back of periodic table. Pb(NO 3)2(aq) + Na 2 Cr. O 4(aq) Pb. Cr. O 4(s) + 2 Na. NO 3(aq) Check to see if one of the products does not dissolve in water (an “s” on the solubility table). If so, that substance is the precipitate and the rxn occurs. If both products are soluble, no rxn occurs.

Predict the products and balance the equations for the following rxns; if no reaction occurs write “no rxn” 1. Al. Cl 3(aq) + Na 3 PO 4(aq) Al. PO 4 (s)+ 3 Na. Cl (aq) 2. KNO 3(aq) + Mg. Br 2(aq) No Rxn 3. 2 Na. Cl(aq) + Pb(NO 3)2(aq) 4. KNO 3(aq) + Ca. Cl 2(aq) 5. Ba. I 2(aq) + Na 2 CO 3(aq) 6. Cu. SO 4(aq) + K 3 PO 4(aq) 7. (NH 4)2 S(aq) + Zn. Cl 2(aq) Pb. Cl 2(s) + Na. NO 3(aq) No Rxn Ba. CO 3(s) + 2 Na. I(aq)

2. A double replacement rxn occurs if one of the products is water (H 2 O). Ex: HCl(aq) + Na. OH(aq) H 2 O(l) + Na. Cl(aq) 2 HNO 3(aq) + Mg(OH)2(s) H 2 O(l) + 2 Mg(NO 3)2(aq) (these rxns occur when an acid reacts with a base – they’re called neutralization rxns) Predict the products and balance the following rxn: H 2 SO 4(aq) + KOH(aq)

3. A double replacement rxn occurs if one of the products is a gas. Ex: 2 HCl(aq) + Na 2 CO 3(aq) H 2 CO 3(aq) + 2 Na. Cl(aq) 2 HCl(aq) + Na 2 CO 3(aq) H 2 O(l) +CO 2(g) + 2 Na. Cl(aq) *metal carbonates react with acids to produce H 2 O, CO 2, and a salt. Predict the products and balance the following rxn: H 2 SO 4(aq) + Ca. CO 3(aq)