Types of reactions Combination Decomposition Single Replacement Double
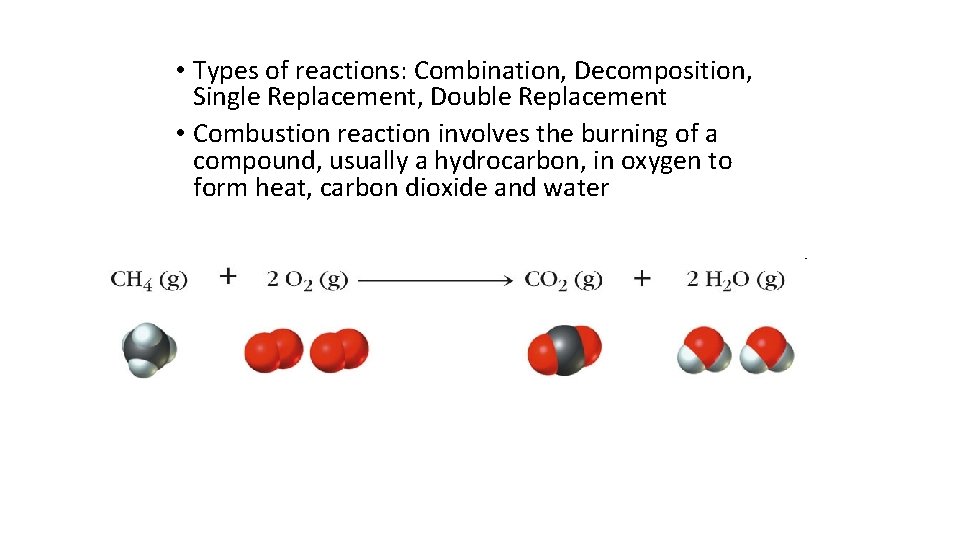
• Types of reactions: Combination, Decomposition, Single Replacement, Double Replacement • Combustion reaction involves the burning of a compound, usually a hydrocarbon, in oxygen to form heat, carbon dioxide and water

Aqueous Solutions • Many times, the chemicals we are reacting together are dissolved in water. • Mixtures of a chemical dissolved in water are called aqueous solutions. • Dissolving the chemicals in water helps them to react together faster. • The water separates the chemicals into individual molecules or ions. • The separate, free-floating particles come in contact more frequently so the reaction speeds up. Tro's "Introductory Chemistry", Chapter 7 2
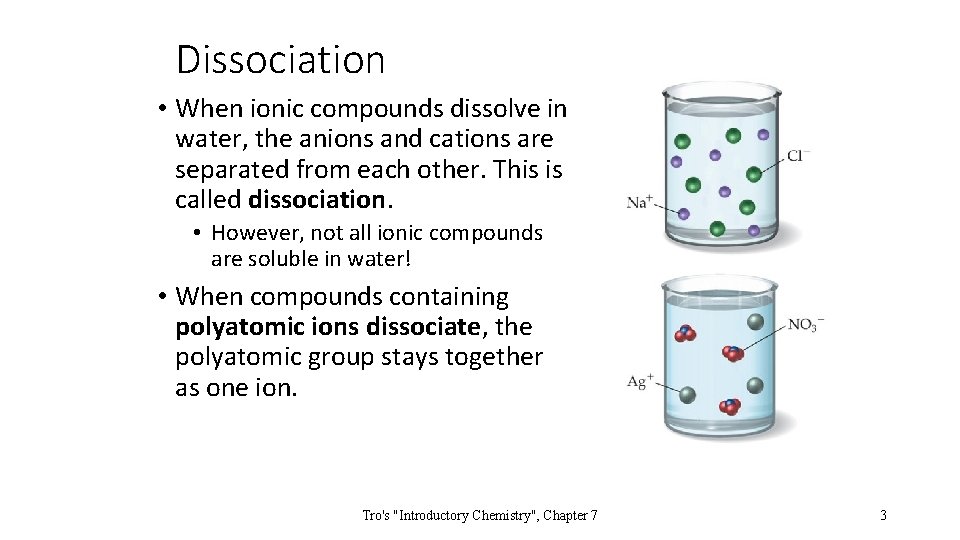
Dissociation • When ionic compounds dissolve in water, the anions and cations are separated from each other. This is called dissociation. • However, not all ionic compounds are soluble in water! • When compounds containing polyatomic ions dissociate, the polyatomic group stays together as one ion. Tro's "Introductory Chemistry", Chapter 7 3
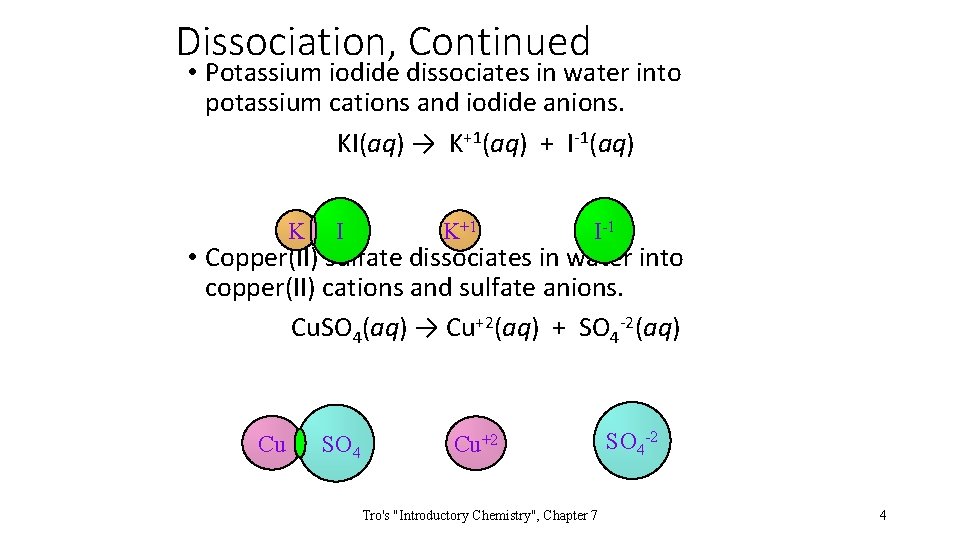
Dissociation, Continued • Potassium iodide dissociates in water into potassium cations and iodide anions. KI(aq) → K+1(aq) + I-1(aq) K I K+1 I-1 • Copper(II) sulfate dissociates in water into copper(II) cations and sulfate anions. Cu. SO 4(aq) → Cu+2(aq) + SO 4 -2(aq) Cu SO 4 Cu+2 Tro's "Introductory Chemistry", Chapter 7 SO 4 -2 4
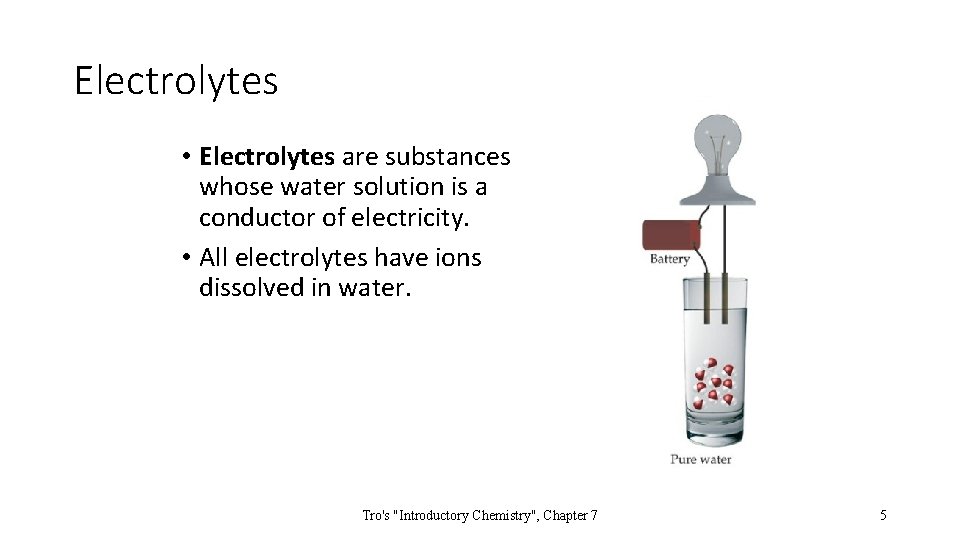
Electrolytes • Electrolytes are substances whose water solution is a conductor of electricity. • All electrolytes have ions dissolved in water. Tro's "Introductory Chemistry", Chapter 7 5

When Will a Salt Dissolve? • A compound is soluble in a liquid if it dissolves in that liquid. • Na. Cl is soluble in water, but Ag. Cl is not. • A compound is insoluble if a significant amount does not dissolve in that liquid. • Ag. Cl is insoluble in water. • Though there is a very small amount dissolved, but not enough to be significant. Tro's "Introductory Chemistry", Chapter 7 6

Predicting Whether a Reaction Will Occur in Aqueous Solution “Forces” that drive a reaction: • • • Formation of a solid. Formation of water. Formation of a gas. Transfer of electrons. When chemicals (dissolved in water) are mixed and one of the above-noted forces occur, the reaction will generally happen. Tro's "Introductory Chemistry", Chapter 7 7
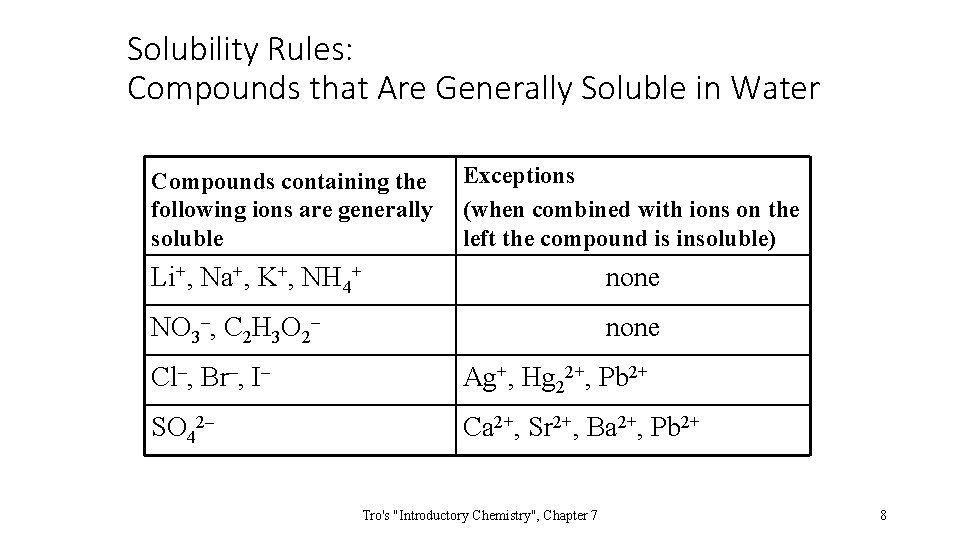
Solubility Rules: Compounds that Are Generally Soluble in Water Compounds containing the following ions are generally soluble Exceptions (when combined with ions on the left the compound is insoluble) Li+, Na+, K+, NH 4+ none NO 3–, C 2 H 3 O 2– none Cl–, Br–, I– Ag+, Hg 22+, Pb 2+ SO 42– Ca 2+, Sr 2+, Ba 2+, Pb 2+ Tro's "Introductory Chemistry", Chapter 7 8
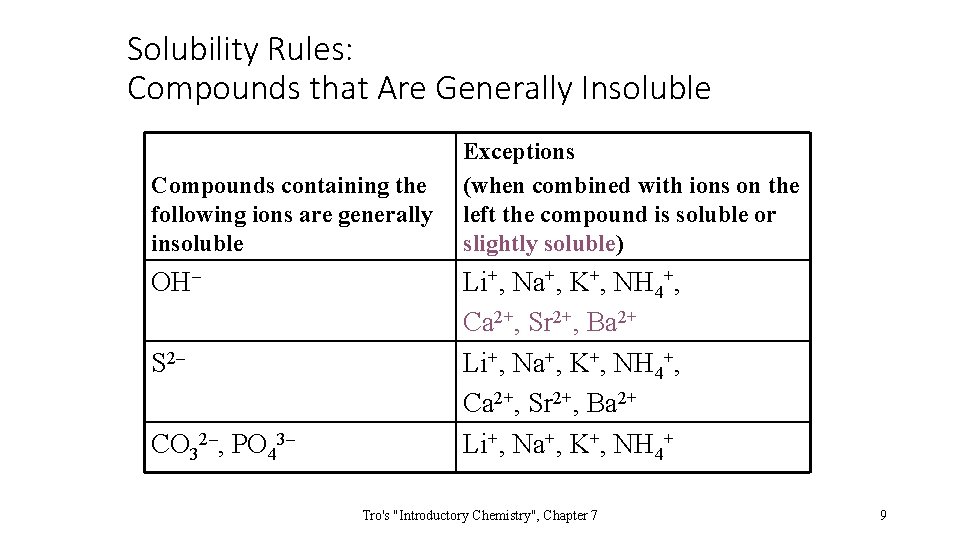
Solubility Rules: Compounds that Are Generally Insoluble Compounds containing the following ions are generally insoluble OH– S 2– CO 32–, PO 43– Exceptions (when combined with ions on the left the compound is soluble or slightly soluble) Li+, Na+, K+, NH 4+, Ca 2+, Sr 2+, Ba 2+ Li+, Na+, K+, NH 4+ Tro's "Introductory Chemistry", Chapter 7 9
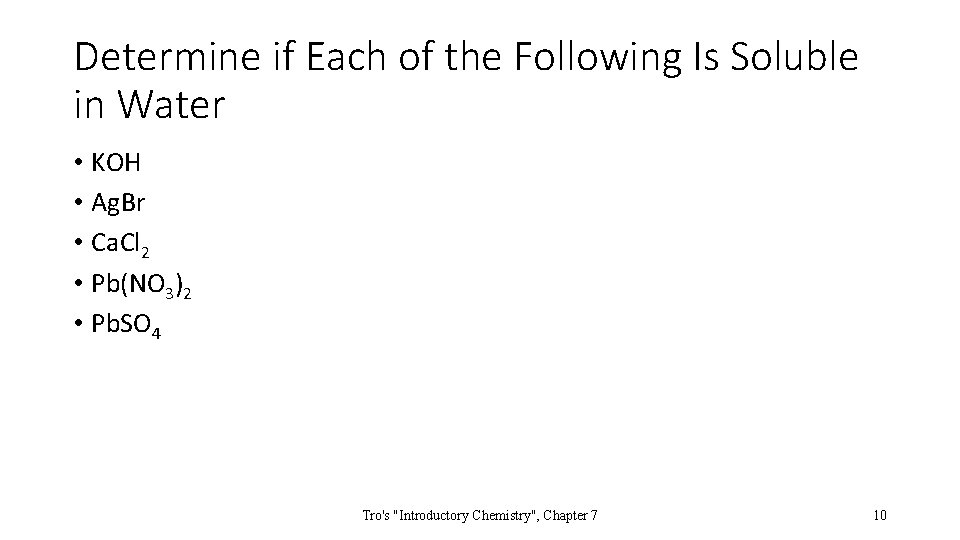
Determine if Each of the Following Is Soluble in Water • KOH • Ag. Br • Ca. Cl 2 • Pb(NO 3)2 • Pb. SO 4 Tro's "Introductory Chemistry", Chapter 7 10
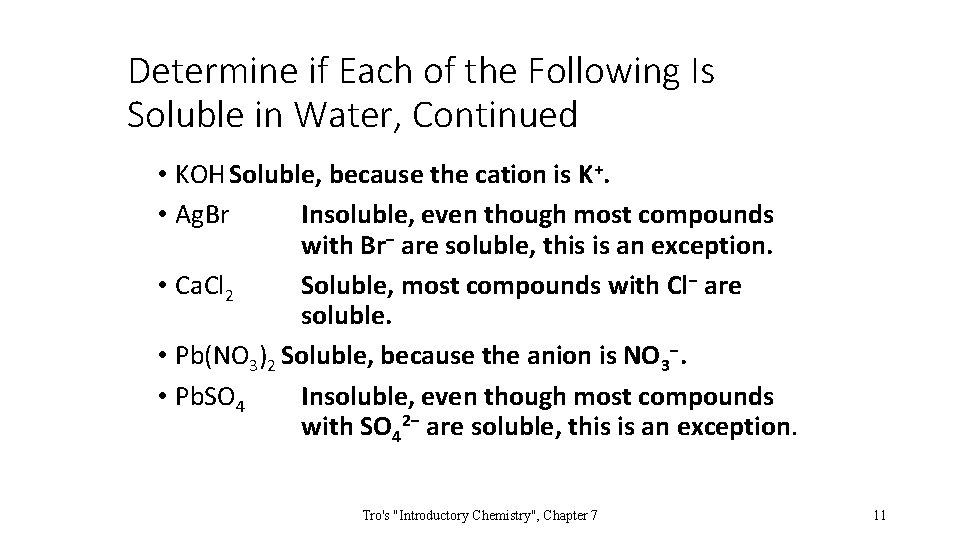
Determine if Each of the Following Is Soluble in Water, Continued • KOH Soluble, because the cation is K+. • Ag. Br Insoluble, even though most compounds with Br− are soluble, this is an exception. • Ca. Cl 2 Soluble, most compounds with Cl− are soluble. • Pb(NO 3)2 Soluble, because the anion is NO 3−. • Pb. SO 4 Insoluble, even though most compounds with SO 42− are soluble, this is an exception. Tro's "Introductory Chemistry", Chapter 7 11

Using the Solubility Rules to Predict an Ionic Compound’s Solubility in Water • First check the cation: If it is Li+, Na+, K+, or NH 4+, then the compound will be soluble in water. • Regardless of the anion. • If the cation is not Li+, Na+, K+, or NH 4+, then follow the rule for the anion. • If a rule says the compounds are mostly soluble, then the exceptions are insoluble. • If a rule says the compounds are mostly insoluble, then the exceptions are soluble. • Note: slightly soluble insoluble. Tro's "Introductory Chemistry", Chapter 7 12

Precipitation Reactions, Continued 2 KI(aq) + Pb(NO 3)2(aq) 2 KNO 3(aq) + Pb. I 2(s) Tro's "Introductory Chemistry", Chapter 7 13

No Precipitate Formation = No Reaction KI(aq) + Na. Cl(aq) KCl(aq) + Na. I(aq) All ions still present, no reaction. Tro's "Introductory Chemistry", Chapter 7 14
Practice–Predict the Products and Balance the Equation • KCl(aq) + Ag. NO 3(aq) • Na 2 S(aq) + Ca. Cl 2(aq) Tro's "Introductory Chemistry", Chapter 7 15

Practice—Write an Equation for the Reaction that Takes Place when an Aqueous Solution of (NH 4)2 SO 4 is Mixed with an Aqueous Solution of Pb(C 2 H 3 O 2)2. Tro's "Introductory Chemistry", Chapter 7 16

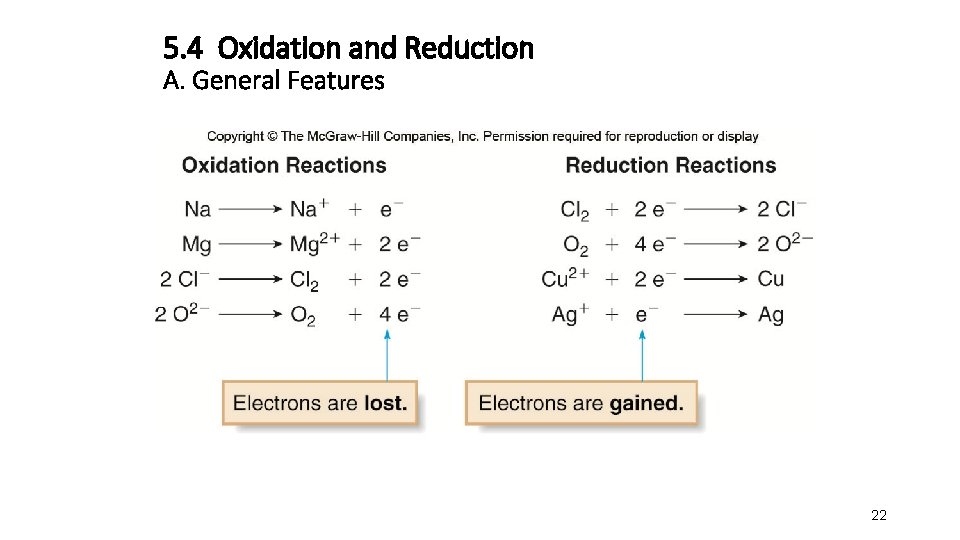
6. Oxidation and Reduction A. General Features • Oxidation is the loss of electrons from an atom. • Reduction is the gain of electrons by an atom. • Both processes occur together in a single reaction called an oxidation−reduction or redox reaction. • A redox reaction always has two components, one that is oxidized and one that is reduced. • A redox reaction involves the transfer of electrons from one element to another. 17
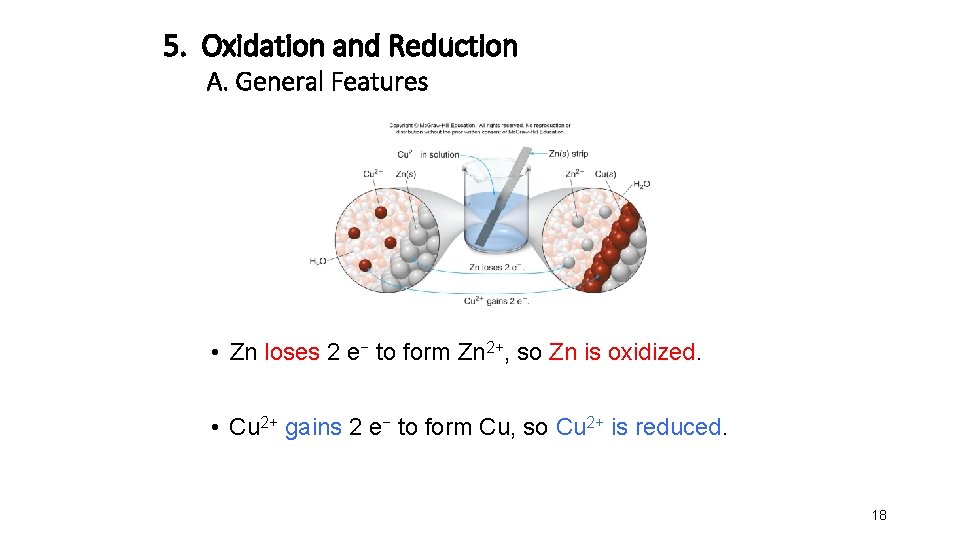
5. Oxidation and Reduction A. General Features • Zn loses 2 e− to form Zn 2+, so Zn is oxidized. • Cu 2+ gains 2 e− to form Cu, so Cu 2+ is reduced. 18
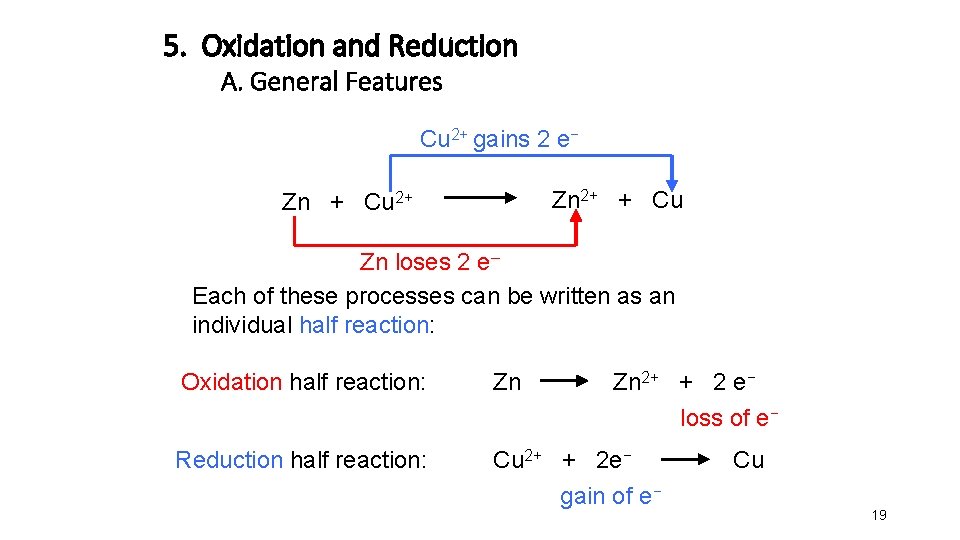
5. Oxidation and Reduction A. General Features Cu 2+ gains 2 e− Zn 2+ + Cu Zn + Cu 2+ Zn loses 2 e– Each of these processes can be written as an individual half reaction: Oxidation half reaction: Zn Zn 2+ + 2 e− loss of e− Reduction half reaction: Cu 2+ + 2 e− gain of e− Cu 19
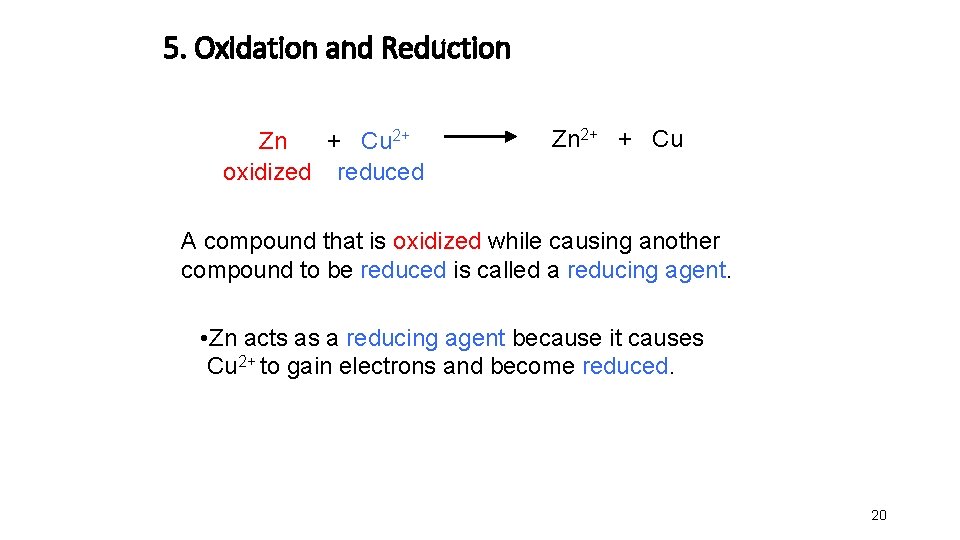
5. Oxidation and Reduction Zn + Cu 2+ oxidized reduced Zn 2+ + Cu A compound that is oxidized while causing another compound to be reduced is called a reducing agent. • Zn acts as a reducing agent because it causes Cu 2+ to gain electrons and become reduced. 20
5. Oxidation and Reduction Zn + Cu 2+ oxidized reduced Zn 2+ + Cu A compound that is reduced while causing another compound to be oxidized is called an oxidizing agent. • Cu 2+ acts as an oxidizing agent because it causes Zn to lose electrons and become oxidized. 21
5. 4 Oxidation and Reduction A. General Features 22
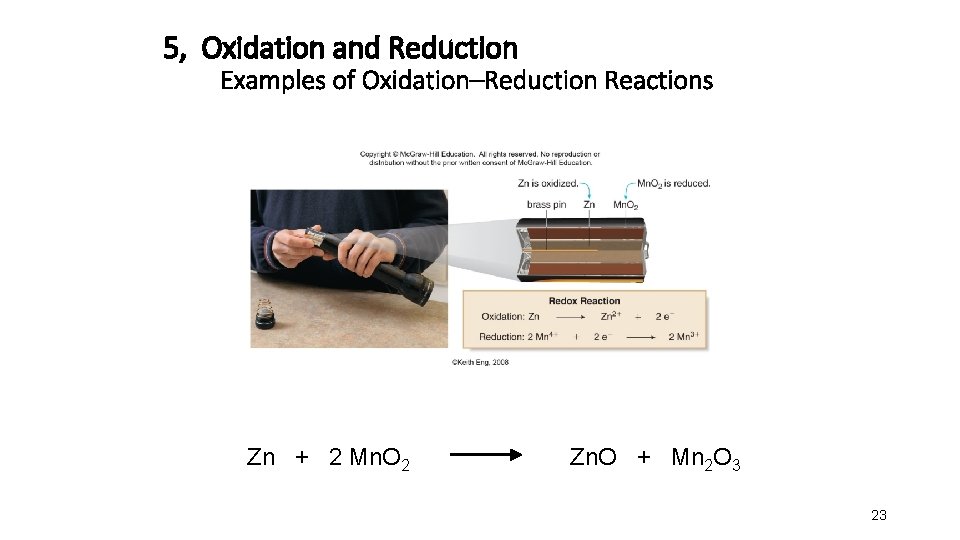
5, Oxidation and Reduction Examples of Oxidation–Reduction Reactions Zn + 2 Mn. O 2 Zn. O + Mn 2 O 3 23
- Slides: 23