Types of Reactions Can you identify the type














- Slides: 21

Types of Reactions Can you identify the type of reaction and predict what gets made?

Types of Chemical Reactions • There are thousands of different chemical reactions. • 4 Major Types (Patterns) – Synthesis – Decomposition – Single Displacement – Double Displacement

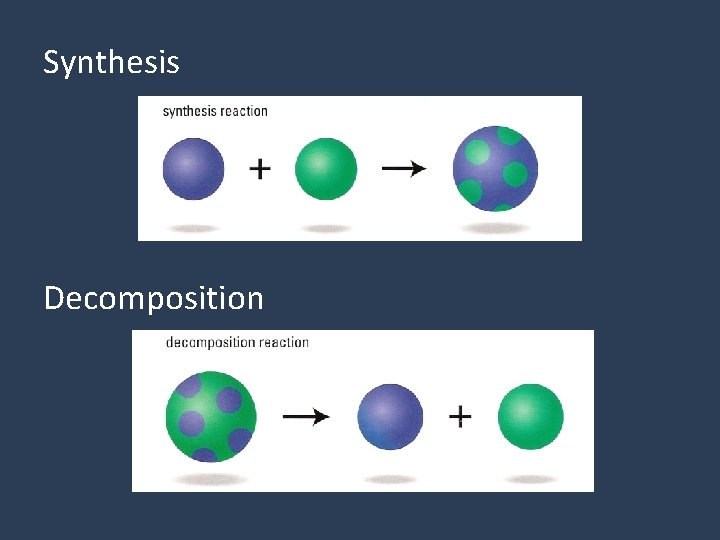
1. Synthesis • Two reactants combine to make a new substance. X + Y ie. Na + Cl 2 H 2 O XY (When 2 become 1. )

Synthesis Tom Katie Tomcat

Synthesis Rxn Real Life: Table Salt

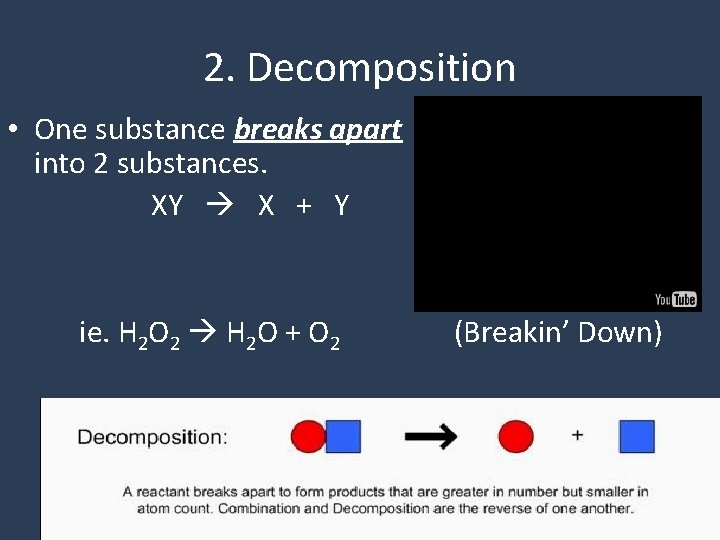
2. Decomposition • One substance breaks apart into 2 substances. XY X + Y ie. H 2 O 2 H 2 O + O 2 (Breakin’ Down)

Decomposition Bennifer Ben Jennifer

Decomposition Demo: Peroxide Marshmallow Experiment Peroxide in 100 m. L grad cylinder placed in large container. Add hand soap and small amount KI

Decomposition Rxn Real Life: Ammonium Nitrate (Fertilizer) Carbonic acid (Pop Fizz)

Is each reaction synthesis or decomposition? 1. Ag 2 CO 3 Ag 2 O + CO 2 Decomposition 2. P 4 + O 2 P 4 O 10 Synthesis 3. Fe + O 2 Fe 2 O 3 Synthesis 4. KCl. O 3 KCl + O 2 Decomposition

Synthesis Decomposition

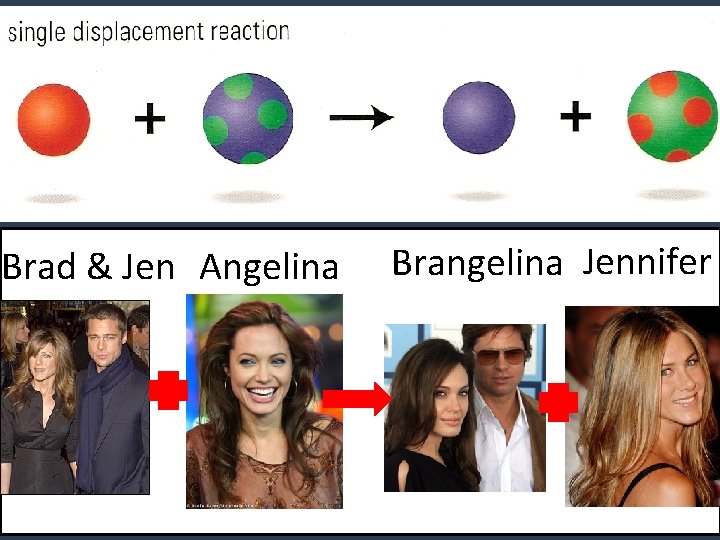
3. Single Displacement • One element takes the place (displaces) of another element in a compound. Like stealing a dance partner. A + BX AX + B ie. HCl + Zn H 2 + Zn. Cl 2

Brad & Jen Angelina Brangelina Jennifer

Single Displacement Rxn Real Life: 2 Ag. NO 3(aq) + Cu(s) Cu(NO 3)2(aq) + 2 Ag(s)

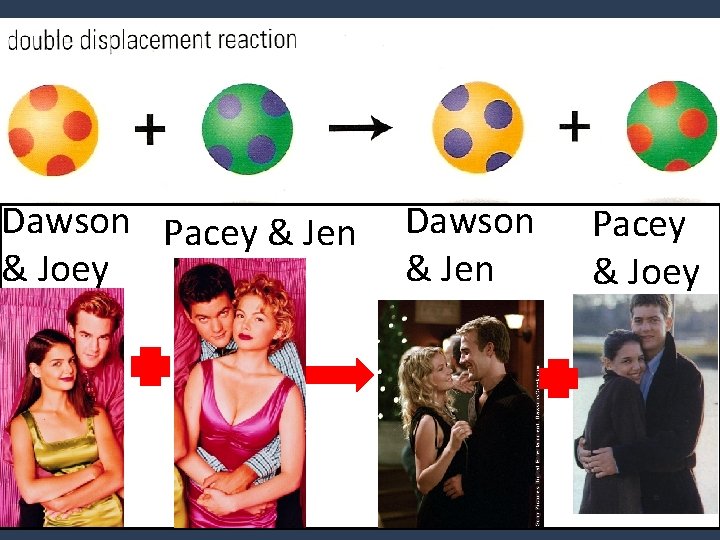
4. Double Displacement • Cations of two compounds exchange places to form 2 new compounds. Like switching dance partners. AB + CD AD + CB ie. Na. OH + HCl Na. Cl + H 2 O

Dawson Pacey & Jen & Joey Dawson & Jen Pacey & Joey

Double Displacement Rxn Real Life: Na. OH(aq) + HCl(aq) Na. Cl(aq) + H 2 O(l)

Double Displacement Demo: Lead Iodide production Add a few drops of Pb(NO 3)2 to some KI. Equation: Pb(NO 3)2 + KI Pb. I 2 + KNO 3

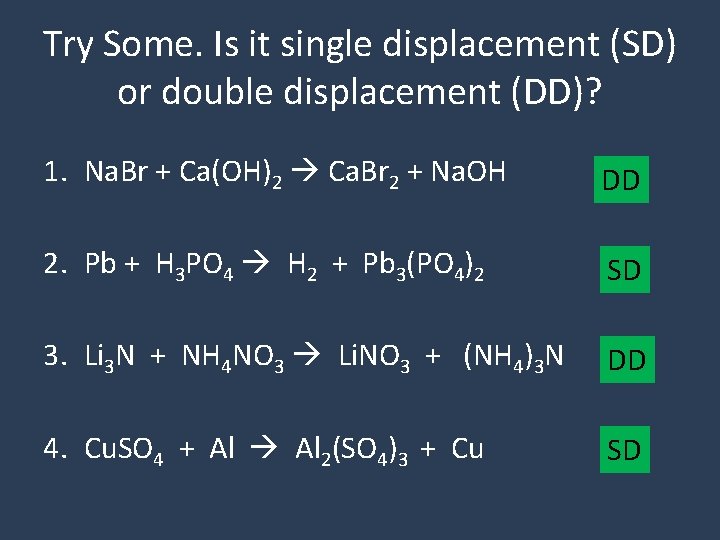
Try Some. Is it single displacement (SD) or double displacement (DD)? 1. Na. Br + Ca(OH)2 Ca. Br 2 + Na. OH DD 2. Pb + H 3 PO 4 H 2 + Pb 3(PO 4)2 SD 3. Li 3 N + NH 4 NO 3 Li. NO 3 + (NH 4)3 N DD 4. Cu. SO 4 + Al 2(SO 4)3 + Cu SD

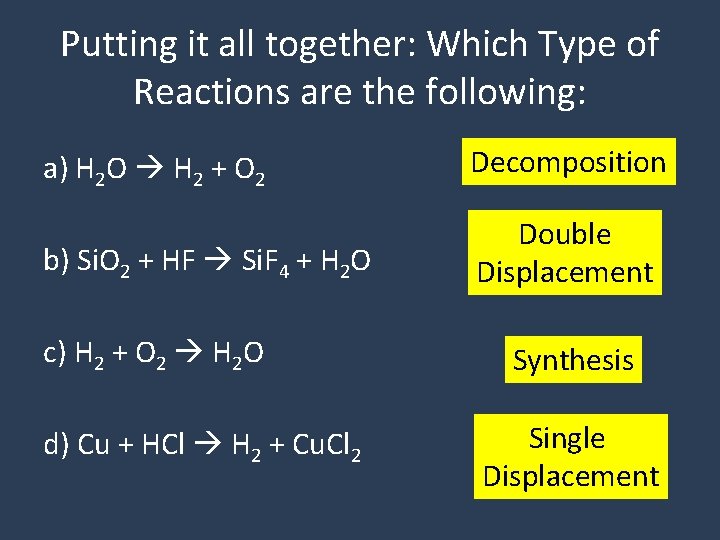
Putting it all together: Which Type of Reactions are the following: a) H 2 O H 2 + O 2 Decomposition b) Si. O 2 + HF Si. F 4 + H 2 O Double Displacement c) H 2 + O 2 H 2 O d) Cu + HCl H 2 + Cu. Cl 2 Synthesis Single Displacement

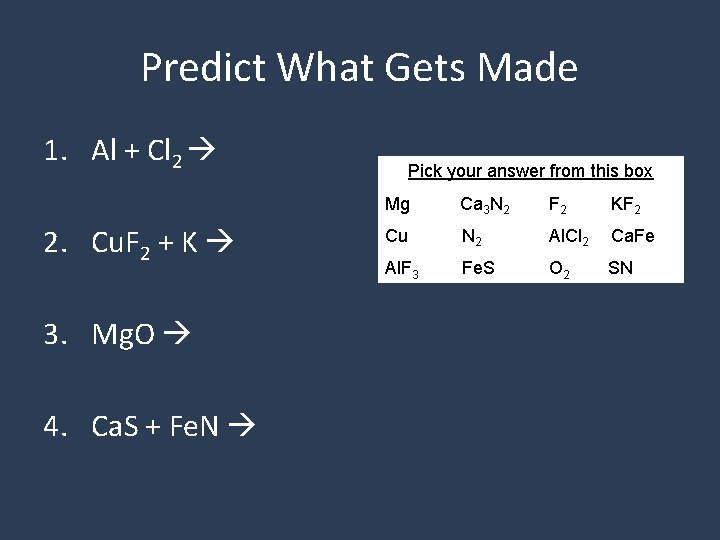
Predict What Gets Made 1. Al + Cl 2 2. Cu. F 2 + K 3. Mg. O 4. Ca. S + Fe. N Pick your answer from this box Mg Ca 3 N 2 F 2 KF 2 Cu N 2 Al. Cl 2 Ca. Fe Al. F 3 Fe. S O 2 SN